Development of a Technology Enabling the Fabrication of Rechargeable Magnesium Batteries in a Dry Air Atmosphere
—Artificial Zinc Coating Effectively Blocks Oxygen Permeation—
2023.05.16
National Institute for Materials Science (NIMS)
A National Institute for Materials Science (NIMS) research team has identified the culprit behind the deactivation of electrochemical reactions within magnesium metal anodes—a rechargeable magnesium battery component—when they are exposed to dry air.
Abstract
- A National Institute for Materials Science (NIMS) research team has identified the culprit behind the deactivation of electrochemical reactions within magnesium metal anodes—a rechargeable magnesium battery component—when they are exposed to dry air. The team then developed an artificial protective anode coating able to prevent electrochemical deactivation. Putting this fundamental technology into practical use may potentially enable the manufacture of rechargeable magnesium batteries using existing lithium-ion battery production lines.
- Rechargeable magnesium batteries—composed entirely of abundant resources, including magnesium—potentially have higher energy densities than lithium-ion batteries. Research and development are underway with the vision of using them as large-capacity storage batteries. However, the use of magnesium metal also has a disadvantage: when it comes into contact with oxygen and moisture, an oxide layer forms on its surface, preventing electrochemical reactions from occurring within it. For this reason, the production, storage and evaluation of rechargeable magnesium batteries need to be performed in an inert gas atmosphere (e.g., argon and nitrogen) from which ambient air needs to be completely removed. Creating such conditions is very expensive and working under them is very cumbersome. These difficulties have seriously hindered efforts to put rechargeable magnesium batteries into practical use.
- This NIMS research team recently discovered that electrochemical deactivation within an air-exposed magnesium metal anode is caused by very high electrical resistance which develops at the three-phase boundary of the electrolyte, the oxygen dissolved in it and the magnesium anode. Following this discovery, the team succeeded in preventing the oxidation of the magnesium metal and deactivation of electrochemical reactions within it even in the presence of dry air. This was achieved by forming an artificial zinc coating on the surface of the magnesium metal anode using galvanic ion-exchange reactions, which prevents oxygen from coming into contact with the magnesium metal. This world-first achievement is academically, industrially and commercially significant as it may enable the production of rechargeable magnesium batteries in dry rooms, greatly facilitating efforts to put them into practical use.
- In addition to these achievements, the research team is also developing a high-performance electrolyte and a magnesium metal anode material for use in rechargeable magnesium batteries. In future research, the team plans to expedite its cathode material R&D in the hope of accelerating the development of practical rechargeable magnesium batteries.
This project was carried out by a research team led by Toshihiko Mandai (Senior Researcher, Rechargeable Battery Materials Group, Research Center for Energy and Environmental Materials, NIMS). - This work was conducted as part of the Advanced Battery Collaboration (JPMJPF2016) research supported by the Japan Science and Technology Agency (JST)’s Program on Open Innovation Platforms for Industry-Academia Co-creation (COI-NEXT). It was also supported by the JSPS Grants-in-Aid for Scientific Research (grant number: 21K05263).
- This research was published in the May 14, 2023 issue (volume 11, number 18) of the Journal of Materials Chemistry A, an online journal of the Royal Society of Chemistry, on April 10, 2023, local time. In addition, this research was selected as a motif for the back cover of this issue.
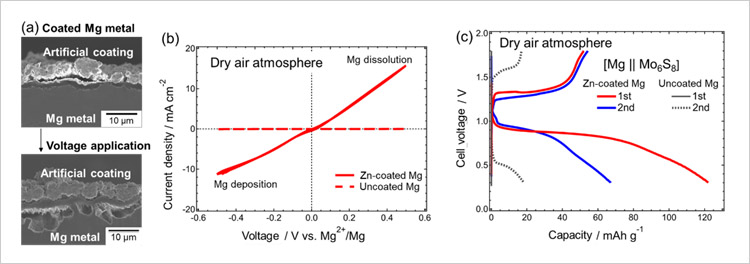
Figure. (a) Electron microscope cross-sectional images of an artificially coated magnesium metal electrode. These images demonstrate that the electrode coating does not interfere with the dissolution of the enclosed magnesium metal when a voltage is applied to the electrode. (b) Comparison of current-voltage response between zinc-coated and uncoated magnesium metal electrodes in dry air atmospheres. (c) Comparison of the charge-discharge profiles of rechargeable magnesium batteries equipped with either zinc-coated or uncoated magnesium metal anodes in dry air atmospheres.
Related files
- Research Center for Energy and Environmental Materials
- Center for Advanced Battery Collaboration
Contact information
(Regarding this research)
-
Toshihiko Mandai
Senior Researcher
Rechargeable Battery Materials Group
Battery and Cell Materials Field
Research Center for Energy and Environmental Materials
Elemental Strategy Team
Center for Advanced Battery Collaboration
National Institute for Materials Science
Tel: +81-29-860-4464
E-Mail: MANDAI.Toshihiko=nims.go.jp
(Please change "=" to "@")
(General information)
-
Public Relations Office
National Institute for Materials Science
Tel: +81-29-859-2026
Fax: +81-29-859-2017
E-Mail: pressrelease=ml.nims.go.jp
(Please change "=" to "@")
Same Keywords
-
Development of a Model Capable of Predicting the Cycle Lives of High-Energy-Density Lithium-Metal Batteries
(battery)
2024.07.24
-
Synthesis of Carbon Nanosheets with Numerous Nanopores
(battery)
2022.06.03
-
Development of a Lithium-Air Battery with an Energy Density over 500 Wh/kg
(battery)
2021.12.15
Recent Press Release
-
No Need for Rare Earths or Liquid Helium! Cryogenic Cooling Material Composed Solely of Abundant Elements
2025.12.24
-
Novel Materials Design Approach Achieves a Giant Cooling Effect and Excellent Durability in Magnetic Refrigeration Materials
2025.12.19
-
Multiple Autonomous AI Systems Spontaneously Collaborate to Advance Materials Research
2025.12.10
