Chemical Crossover Accelerates Degradation of Lithium Electrode in High Energy Density Rechargeable Lithium–Oxygen Batteries
—Use of Lightweight Protective Layer Greatly Extends Battery Cycle Life—
2023.01.31
National Institute for Materials Science (NIMS)
Japan Science and Technology Agency
SoftBank Corp.
Ohara Inc.
NIMS, SoftBank Corp. and Ohara Inc. jointly identified the detailed mechanisms behind the degradation of high-energy-density lithium–oxygen batteries.
Abstract
- The National Institute for Materials Science (NIMS), SoftBank Corp. and Ohara Inc. jointly identified the detailed mechanisms behind the degradation of high-energy-density lithium–oxygen batteries. Analytical studies using a combination of advanced techniques reveals that lithium negative electrode severely degraded associated with the progress cycle, resulting in the short cycle life of lithium–oxygen batteries. By using a lightweight protective layer for lithium electrodes, the cycle life of lithium–oxygen batteries largely extended without diminishing their high energy density (measured in Wh/kg).
- Lithium–oxygen batteries have the potential to be the ultimate rechargeable batteries: they are lightweight and high capacity, with theoretical energy densities per unit weight several times that of currently available lithium ion batteries. Because of these potential advantages, there is growing attention for use of this battery system for a wide range of technologies, such as drones, electric vehicles and household electricity storage systems. NIMS has been carrying out basic research on lithium–oxygen batteries with support from the ALCA-SPRING program (ALCA: Advanced Low Carbon Technology Research and Development Program, SPRING: Specially Promoted Research for Innovative Next-Generation Batteries). This program has been funded by the Japan Science and Technology Agency (JST) with the goal of accelerating large-capacity rechargeable battery R&D. In 2018, NIMS and SoftBank co-founded their Advanced Technologies Development Center to conduct research with the aim of putting lithium–oxygen batteries into practical use in mobile phone base stations, the Internet of Things (IoT), HAPS (high altitude platform stations) and other technologies. In 2021, the Center succeeded in developing a lithium–oxygen battery with an energy density of nearly 500 Wh/kg, greatly surpassing the energy densities of previously developed lithium–oxygen batteries. However, the life of this battery was only 10 charge/discharge cycles or less, which needed to be addressed before it could be put into practical use.
- Using a combination of various advanced analytical techniques previously developed by this research team, the team found that a lithium–oxygen battery’s metallic lithium negative electrode degrades rapidly while being subjected to charge/discharge cycles, causing significant overpotential in the battery and shortening its cycle life. This result contradicts the conventional belief that cycle-life-reducing overpotential is induced by electrochemical reactions taking place in the oxygen positive electrode. To prevent the degradation of the metallic lithium negative electrode, the research team also developed a light and flexible solid electrolyte 6 μm in thickness and integrated it into a lithium–oxygen battery as a protective layer. As a result, the battery’s cycle life was significantly extended without compromising its high energy density per unit weight.
- The research team hopes to expedite the development of practical lithium–oxygen batteries at the NIMS-SoftBank Advanced Technologies Development Center by developing new battery materials and incorporating them into the batteries.
- This project was carried out by a research team led by Shoichi Matsuda (Senior Researcher, NIMS), Manai Ono (Postdoctoral Researcher, NIMS) and Kohei Uosaki (Research Fellow, NIMS; also Director, NIMS-SoftBank Advanced Technologies Development Center). This work was conducted as part of R&D efforts at the NIMS-SoftBank Advanced Technologies Development Center with support from the JST ALCA-SPRING program.
- This research was published in the online version of Advanced Energy Materials on January 30, 2023, Japan Time.
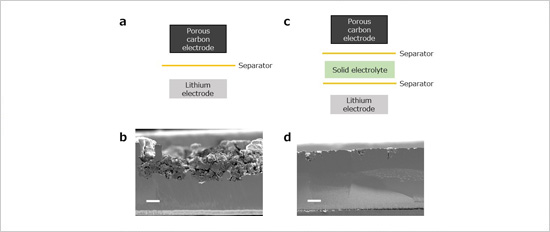
Figure. (a) Schematic illustration of a lithium–oxygen battery without a protective layer. (b) Cross-sectional SEM image of lithium negative electrodes taken out from the cell after charge/discharge cycles without a protective layer. The thickness of the electrode diminished from the initial 100 μm down to about 50 μm as it severely degraded. (c) Schematic illustration of a lithium–oxygen battery with a protective layer. (d) Cross-sectional SEM image of lithium negative electrodes taken out from the cell after charge/discharge cycles with a protective layer. Its initial 100-μm thickness remained mostly unchanged, indicating that the protective layer is effective in reducing electrode degradation. The white scale bars represent 20 μm in length.
Related files
- Center for Green Research on Energy and Environmental Materials
Contact information
(Regarding this research)
-
Shoichi Matsuda
Senior Researcher
Solid-State Battery Group
Center for Green Research on Energy and Environmental Materials
National Institute for Materials Science
Tel: +81-29-860-4637
E-Mail: MATSUDA.Shoichi=nims.go.jp
(Please change "=" to "@")
(Regarding JST programs)
-
Shinichi Kato
Department of R&D for Future Creation
Japan Science and Technology Agency
K's Gobancho, 7 Gobancho, Chiyoda-ku, Tokyo 102-0076, Japan
Tel: +81-3-3512-3543
E-Mail: alca=jst.go.jp
(Please change "=" to "@")
(General information)
-
Public Relations Office
National Institute for Materials Science
Tel: +81-29-859-2026
Fax: +81-29-859-2017
E-Mail: pressrelease=ml.nims.go.jp
(Please change "=" to "@") -
Public Relations Division
Japan Science and Technology Agency
5-3 Yonbancho, Chiyoda-ku, Tokyo 102-8666, Japan
Tel: +81-3-5214-8404
Fax: +81-3-5214-8432
E-Mail: jstkoho=jst.go.jp
(Please change "=" to "@") -
Tatsuya Sakaizumi
Ohara Inc.
1-15-30 Oyama, Chuo-ku, Sagamihara-shi, Kanagawa 252-5286, Japan
Tel: +81-42-718-2698
Fax: +81-42-774-1799
E-Mail: sakaizumi=ohara-inc.co.jp
(Please change "=" to "@")
Same Keywords
-
Identification of a Major Factor Influencing the Cycle Life of Practical Li-O2 Batteries
(cycle life,JST,SoftBank)
2020.12.02
-
Development of 1-Wh-Class Stacked Lithium-Air Cells
(lithium,batteries)
2025.09.19
-
Development of a Lithium-Air Battery with an Energy Density over 500 Wh/kg
(lithium,JST)
2021.12.15
Recent Press Release
-
No Need for Rare Earths or Liquid Helium! Cryogenic Cooling Material Composed Solely of Abundant Elements
2025.12.24
-
Novel Materials Design Approach Achieves a Giant Cooling Effect and Excellent Durability in Magnetic Refrigeration Materials
2025.12.19
-
Multiple Autonomous AI Systems Spontaneously Collaborate to Advance Materials Research
2025.12.10
