Formation of Black Rust Accelerates Bacteiral Iron Corrosion
—Discovery of Bacteria’s Ability to Extract Electrons without a Special Enzyme May Promote the Development of New Anti-Corrosion Materials—
2020.02.14
National Institute for Materials Science (NIMS)
NIMS, CSIRO and RIKEN have jointly discovered that highly electrically conductive black rust formed by iron-corroding bacteria increases the metabolic activity of the bacteria and accelerates microbially influenced iron corrosion. This finding contradicts the previously held belief that black rust is an unusable byproduct for its producer, the iron-corroding bacteria. This result may prompt the development of new iron alloy materials capable of decreasing the electrical conductivity of black rust, thereby preventing bacterial corrosion.
Abstract
- NIMS, CSIRO and RIKEN have jointly discovered that highly electrically conductive black rust formed by iron-corroding bacteria increases the metabolic activity of the bacteria and accelerates microbially influenced iron corrosion. This finding contradicts the previously held belief that black rust is an unusable byproduct for its producer, the iron-corroding bacteria. This result may prompt the development of new iron alloy materials capable of decreasing the electrical conductivity of black rust, thereby preventing bacterial corrosion.
- Corrosion of iron infrastructure—including petroleum pipelines—caused by sulfate-reducing bacteria has been a serious problem. Iron reacts with hydrogen sulfide produced metabolically by this bacterium to form iron sulfide (black rust), corroding the iron surface. This bacterial corrosion process is known to advance even after iron surfaces are completely covered by iron sulfide. The reason for this was unknown and no effective anti-corrosion methods were available. This research team previously demonstrated that sulfate-reducing bacteria have special enzymes on their cellular membranes that may be capable of directly extracting electrons from iron surfaces via the conductive iron sulfide covering the surface, thereby accelerating iron corrosion. However, sulfate-reducing bacteria without the enzyme also showed the ability to effectively corrode iron surfaces covered with iron sulfide, suggesting the existence of an electron extraction mechanism that does not require membranous enzymes.
- This research team focused on the electrical conductivity of iron sulfide, a primary component of black rust. Iron sulfide nanoparticles accumulate both within bacterial cells and on their surfaces. The team recently performed in-depth analysis on these nanoparticles formed on bacterial surfaces and found that they have highly electrically conductive crystalline structures. The team then compared the metabolic activity of sulfate-reducing bacteria containing iron sulfide nanoparticles with those not containing them. Only bacteria containing nanoparticles were observed to acquire electrons from an external solid electron source and incorporate nitrogen source into their cells, indicating increased metabolic activity. Although black rust was previously believed to be an unusable byproduct for sulfate-reducing bacteria, these results indicate that it plays an important biochemical function. The results also suggest that sulfate-reducing bacteria without special membranous enzymes can extract electrons from black rust-covered iron materials, thereby accelerating iron corrosion.
- In future studies, we plan to develop environmentally safe technologies to prevent bacterial corrosion of iron, including design of iron alloys that only permit the formation of black rust with poorly electrically conductive structures. The use of such materials may eliminate the need for environmentally harmful bactericides.
- This project was carried out by a research team consisting of Akihiro Okamoto (Independent Scientist, International Center for Materials Nanoarchitectonics (MANA), NIMS), Xiao Deng (Postdoctoral Researcher, MANA, NIMS; currently affiliated with CSIRO) and Naoshi Dohmae (Unit Leader, Center for Sustainable Resource Science, RIKEN).
This work was supported in part by JSPS Grant-in-Aid for Young Scientists (A) (project number 17H04969) and other sources.
This research was published in the online version of Angewandte Chemie International Edition as a “very important paper”—an honor given to only 5% of the journal’s papers—on January 29, 2020.
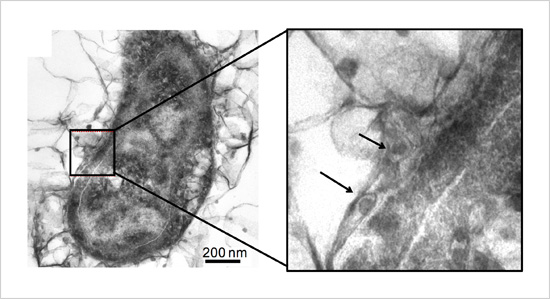
Figure. Iron sulfide nanoparticles accumulated inside and outside the cellular membrane in an iron-corroding bacterium
Related files
- MANA
Contacts
(Regarding this research)
-
Akihiro Okamoto
Independent Scientist,
International Center for Materials Nanoarchitectonics,
National Institute for Materials Science (NIMS)
Tel: +81-860-4430
E-Mail: okamoto.akihiro=nims.go.jp
(Please change "=" to "@")
(General information)
-
Public Relations Office
National Institute for Materials Science
Tel: +81-29-859-2026
Fax: +81-29-859-2017
E-Mail: pressrelease=ml.nims.go.jp
(Please change "=" to "@")
Recent Press Release
-
Simultaneous Imaging of Intracellular DNA and RNA Using Harmless Light
2025.10.27
-
Development of an AI Device Using Ion Gel and Graphene That Dramatically Streamlines Machine Learning Computations
2025.10.14
-
Demonstrating a Novel Method to Modulate Heat Flow Through the Collective Motion of Spins
2025.10.06
