Ammonia Synthesis at Ambient Pressure without Using Expensive Catalysts
—The New Technique May Reduce the Costs of Chemical Fertilizer Synthesis and Ammonia-Based Hydrogen Storage and Transport—
2017.09.28
National Institute for Materials Science (NIMS)
NIMS discovered a novel ammonia synthesis reaction which can be induced simply by introducing a hydrogen-nitrogen gas mixture into inexpensive liquid sodium. Conventional methods of synthesizing ammonia—an ingredient for chemical fertilizers and other products—at ambient pressure require expensive catalysts or synthesis routes.
(“Synthesis of ammonia using sodium melt,” Fumio Kawamura & Takashi Taniguchi; Scientific Reports 7, Article number: 11578 (2017), doi:10.1038/s41598-017-12036-9)
Abstract
- NIMS discovered a novel ammonia synthesis reaction which can be induced simply by introducing a hydrogen-nitrogen gas mixture into inexpensive liquid sodium. Conventional methods of synthesizing ammonia—an ingredient for chemical fertilizers and other products—at ambient pressure require expensive catalysts or synthesis routes. The technique may enable ammonia synthesis at lower cost.
- More than 170 million tons of ammonia are produced annually as ingredients for chemical fertilizers and other products. Because hydrogen is a potential next-generation energy source and can be safely stored and transported by converting it into ammonia, its demand is expected to increase. The conventional Haber-Bosch process produces ammonia by allowing hydrogen and nitrogen to react at 200-400 atmospheric pressure. The process was developed into an industrial procedure about 100 years ago and is still used today for mass production of ammonia. This method, however, has several issues, such as requiring many chemical conversion steps and high synthetic cost. Therefore, research efforts have been intensified in recent years to develop more affordable methods of synthesizing ammonia at ambient pressure.
- A NIMS research team recently developed a simple ammonia synthesis method which only requires introducing bubbles of a hydrogen-nitrogen gas mixture into inexpensive liquid sodium. The team confirmed that this method synthesized a small amount of ammonia at ambient pressure. Sodium had been thought to be capable of breaking down strong bonds between nitrogen atoms to yield nitrogen radicals or ions for ammonia synthesis. However, sodium reacts with hydrogen, rather than nitrogen, at low temperatures. In this method, sodium was liquefied at 500°C or higher, which presumably prevented sodium from reacting with hydrogen and facilitated its reaction with nitrogen enabling ammonia synthesis.
- This ammonia synthesis method can be implemented at ambient pressure using sodium, an inexpensive substance. Practical application of this method may enable miniaturization of ammonia synthesis devices and more economical synthesis. In future studies, we will seek to increase the ammonia synthesis efficiency of this method by pressurizing gas mixture bubbles and pursuing other options in verification tests.
- This study was conducted by a NIMS research team led by Fumio Kawamura (Senior Researcher, High Pressure Group, Research Center for Functional Materials). The study was published in the September 14, 2017, issue of Scientific Reports online.
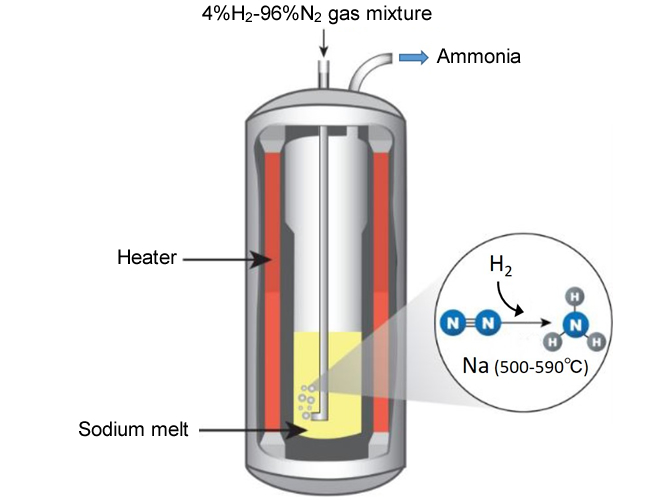
Conceptual diagram showing the process of ammonia synthesis in liquid sodium
Related files
- Research Center for Functional Materials
Contacts
(Regarding this research)
-
Fumio Kawamura
Senior Researcher
High Pressure Group, Research Center for Functional Materials,
National Institute for Materials Science (NIMS)
Tel: +81-29-860-4428
E-Mail: KAWAMURA.Fumio=nims.go.jp
(Please change "=" to "@")
(For general inquiries)
-
Public Relations Office, Planning Division
National Institute for Materials Science
1-2-1 Sengen, Tsukuba, Ibaraki, 305-0047, Japan
Tel: +81-29-859-2026
Fax: +81-29-859-2017
E-Mail: pressrelease=ml.nims.go.jp
(Please change "=" to "@")
