Hidden Cause of Lithium-Rich Cathode Materials’ Low Energy Efficiency Revealed
—Asymmetric Reaction Pathways Are Key—
2023.12.15
National Institute for Materials Science (NIMS)
A research team consisting of the National Institute for Materials Science (NIMS) and SoftBank Corp. has found that voltage hysteresis in Li2RuO3—a high-energy-density rechargeable battery cathode material—is caused by differences in the intermediate crystalline phases formed during charge and discharge processes.
Abstract
- A research team consisting of the National Institute for Materials Science (NIMS) and SoftBank Corp. has found that voltage hysteresis in Li2RuO3—a high-energy-density rechargeable battery cathode material—is caused by differences in the intermediate crystalline phases formed during charge and discharge processes. Voltage hysteresis is a phenomenon detrimental to lithium (Li)-ion batteries in which discharge voltage becomes significantly lower than charge voltage. These results revealed a voltage-hysteresis-causing mechanism inconsistent with conventional theory.
- Li-rich electrode materials are capable of storing larger amounts of Li ions than conventional Li-ion battery cathode materials (e.g., LiCoO2) and Li ions can be stably extracted from and inserted into them. In addition, the energy capacity of these materials (> 300 mAh/g) is approximately twice that of conventional cathode materials. Because of these desirable characteristics, Li-rich electrode materials have been researched as viable candidates for next-generation, high-energy-density Li-ion battery cathode materials. They also have a disadvantage, however: poor charge/discharge energy efficiency due to large voltage hysteresis occurring during charge and discharge.
- It has been widely accepted by the scientific community that voltage hysteresis in Li-rich electrode materials results from irreversible changes in their crystalline structures during charge and discharge. This research team focused on Li2RuO3 as a model Li-rich electrode material and closely observed changes in its crystalline structure while it was being charged and discharged. Its crystalline structure was found to change reversibly, not irreversibly—it recovered its initial pre-charge crystalline structure by the end of the subsequent discharge. During this charge/discharge cycle, voltage hysteresis was observed in Li2RuO3 despite the absence of irreversible crystalline structure changes—a result contrary to conventional theory. The team then closely analyzed crystalline structure changes in an Li2RuO3 electrode while it was being charged and discharged using various advanced analytical instruments. These analyses revealed a discrepancy in the intermediate crystal phase formed during the charge and discharge processes causing the voltage hysteresis. In other words, voltage hysteresis within a Li-rich electrode material appears to be attributed to different reaction pathways rather than irreversible crystalline structure changes.
- Based on these results, the research team plans to evaluate Li-rich electrode materials while focusing on chemical reaction pathways during charge and discharge cycles in addition to measuring voltage hysteresis. This approach is expected to expedite the development of Li-rich electrode materials that will satisfy both high capacity and high charge/discharge energy efficiency requirements.
- This research was carried out by a research team led by Marcela Calpa (Researcher, NIMS), Kei Kubota (Senior Researcher, NIMS), Shoichi Matsuda (Team Leader, NIMS) and Kazunori Takada (Research Fellow, NIMS; also Director, NIMS-SoftBank Advanced Technologies Development Center) at the NIMS-SoftBank Advanced Technologies Development Center.
- This research was published in Energy Storage Materials, an online journal, on November 6, 2023, Japan Time.
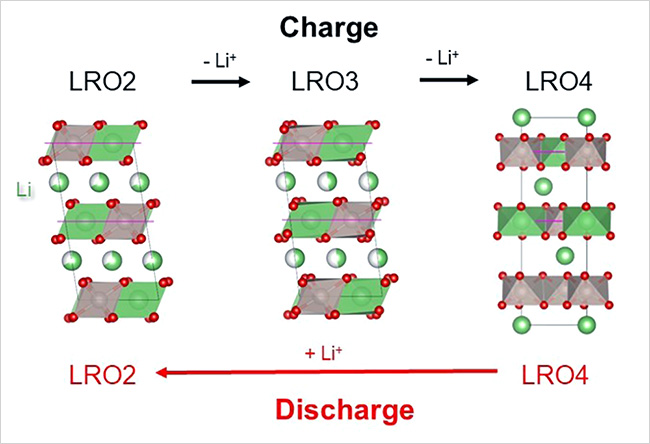
Figure. Asymmetric pathways in crystal structure change during charge/discharge reaction
Related files
- Research Center for Energy and Environmental Materials
Contact information
(Regarding this research)
-
Kazunori Takada
Fellow
Research Center for Energy and Environmental Materials
National Institute for Materials Science
E-Mail: TAKADA.Kazunori=nims.go.jp
(Please change "=" to "@")
(General information)
-
Public Relations Office
National Institute for Materials Science
Tel: +81-29-859-2026
Fax: +81-29-859-2017
E-Mail: pressrelease=ml.nims.go.jp
(Please change "=" to "@")
Recent Press Release
-
No Need for Rare Earths or Liquid Helium! Cryogenic Cooling Material Composed Solely of Abundant Elements
2025.12.24
-
Novel Materials Design Approach Achieves a Giant Cooling Effect and Excellent Durability in Magnetic Refrigeration Materials
2025.12.19
-
Multiple Autonomous AI Systems Spontaneously Collaborate to Advance Materials Research
2025.12.10
