Technique to Characterize Electrical Potential Distribution in Composite Electrodes of Solid State Lithium Ion Batteries
—Major Advancement in Understanding the Cause of High Resistivity at the Electrode–Electrolyte Interfaces, Which Has Been Hindering the Development of High Power Density Batteries—
2016.12.26
(2017.02.14 Update)
National Institute for Materials Science (NIMS)
A NIMS research team succeeded in visualizing the nanoscale change in potential distribution in composite cathode materials of solid state lithium ion batteries before and after charging/discharging the batteries.
Abstract
- A NIMS research team led by senior researcher Nobuyuki Ishida and postdoctoral researcher Hideki Masuda, Surface Characterization Group, Research Center for Advanced Measurement and Characterization (Ishida is also a GREEN leader in the Nano Interface Characterization Group), succeeded in visualizing the nanoscale change in potential distribution in composite cathode materials of solid state lithium ion batteries (SS-LIBs) before and after charging/discharging the batteries. The results from this study may contribute to identifying the cause of high resistivity at the electrode–electrolyte interfaces, which has been hindering the development of high power density SS-LIBs.
- Due to their proven safety and excellent cycle characteristics, SS-LIBs are envisioned as promising next-generation storage batteries. However, because of the higher transfer resistance of lithium ions at the electrode–solid electrolyte interfaces compared to that at the electrode–liquid electrolyte interfaces, it is difficult to increase the power density of SS-LIBs. To understand the origin of interfacial resistivity, modeling was applied to the lithium ion depleted layer (space-charge layer), which forms in solid electrolytes when SS-LIBs are being charged, and to defects at the interfacial layer. To test these hypotheses, it is critical to measure the change in thickness of the space-charge layer, and the change in the distribution of lithium ion concentrations in that layer before and after charging/discharging the batteries. Then, it will be feasible to analyze the correlation between these measurements and interfacial resistivity. However, it had been difficult to measure electrical potential distribution in SS-LIB samples as the samples need to be extracted without compromising the performance of the battery. This had been a major issue preventing researchers from investigating the cause of interfacial resistivity.
- The research team developed a method whereby samples to be measured are cut out from SS-LIBs, the cross-section of the samples is treated, and potential distribution is measured using a scanning probe microscope, all of which are performed under an inert gas atmosphere or in vacuum. Then the team successfully visualized change in potential distribution arising from battery charging/discharging in the composite cathode at the high spatial resolution (≤50 nm) while keeping the battery performance. When SS-LIBs (provided by Taiyo Yuden Co., Ltd.) were evaluated using this method, the results indicated that the area where lithium ion concentrations decreased in the order of micrometers expanded in the solid electrolyte region, and that charging states were locally inhomogeneous.
- This method is applicable to the evaluation of space-charge layers in many types of SS-LIBs, and may contribute to understanding the causes of high interfacial resistivity in SS-LIBs. In addition, this method is also applicable to the evaluation of differences in charging/discharging states for individual active material particles that arise due to non-uniform electrical conductivity distribution in the composite electrode materials. Therefore, the new method may not only contribute to the design of interfaces to improve the performance of SS-LIBs but also apply to various battery analysis techniques including the analysis of causes of battery degradation.
- A part of this study was conducted in conjunction with the project titled “Formation of super-ion conduction path in all-solid-state lithium ion rechargeable battery through design of the crystal phase-interface with hierarchically controlled structures” (Katsuya Teshima, research director), which was carried out to supplement the project “Creation of innovative functional materials with advanced properties by hyper-nano-space design” (Tohru Setoyama, research supervisor), under the Strategic Basic Research Programs (specifically the CREST program) sponsored by the Japan Science and Technology Agency (JST). This study was also funded by the Global Research Center for Environment and Energy based on Nanomaterials Science (GREEN, Koei Uosaki, director-general), which was founded to implement the MEXT-commissioned project titled “Integrated materials development project.” We conducted this research at the facility of the NIMS Battery Research Platform.
- This study, coauthored by NIMS and Taiyo Yuden, was published in the online version of Nanoscale, a journal issued by the Royal Society of Chemistry, on December 21, 2016. (DOI: 10.1039/C6NR07971G)
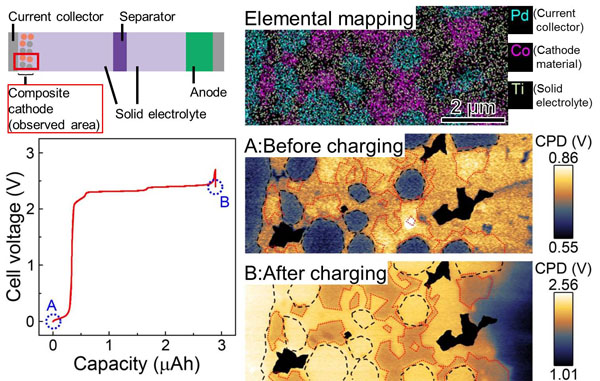
Figure. Electrical potential distribution in composite cathode regions on the cross-section of a solid state lithium ion battery
Related files
- Research Center for Advanced Measurement and Characterization
Contacts
(Regarding this research)
-
Nobuyuki Ishida
Senior Researcher
Surface Characterization Group, RCAMC
National Institute for Materials Science
Tel: +81-29-860-4972
E-Mail: ISHIDA.Nobuyuki=nims.go.jp
(Please change "=" to "@") -
Hideki Masuda
Postdoctoral Researcher
Surface Characterization Group, RCAMC
National Institute for Materials Science
Tel: +81-29-851-3354
E-Mail: MASUDA.Hideki=nims.go.jp
(Please change "=" to "@")
(Regarding public relations)
-
Public Relations Office
National Institute for Materials Science
1-2-1 Sengen, Tsukuba, Ibaraki, 305-0047, JAPAN
Tel: +81-29-859-2026
Fax: +81-29-859-2017
E-Mail: pressrelease=ml.nims.go.jp
(Please change "=" to "@")
Same Keywords
-
Development of a Model Capable of Predicting the Cycle Lives of High-Energy-Density Lithium-Metal Batteries
(battery)
2024.07.24
-
Development of a Technology Enabling the Fabrication of Rechargeable Magnesium Batteries in a Dry Air Atmosphere
(battery)
2023.05.16
-
Synthesis of a Silicon-Integrated Organic Framework Film
(nano)
2022.11.08
Recent Press Release
-
No Need for Rare Earths or Liquid Helium! Cryogenic Cooling Material Composed Solely of Abundant Elements
2025.12.24
-
Novel Materials Design Approach Achieves a Giant Cooling Effect and Excellent Durability in Magnetic Refrigeration Materials
2025.12.19
-
Multiple Autonomous AI Systems Spontaneously Collaborate to Advance Materials Research
2025.12.10
