Cause of the Detrimental Charge Voltage Rise in Lithium-Air Batteries Identified
2020.08.12
National Institute for Materials Science (NIMS)
Japan Science and Technology Agency (JST)
NIMS has found for the first time that a voltage increase that occurs in lithium-air batteries when they are being charged—a major issue preventing their practical use—is strongly and positively correlated with the degree of crystallinity of lithium peroxide (Li2O2), a compound produced during a discharge cycle.
Abstract
- NIMS has found for the first time that a voltage increase that occurs in lithium-air batteries when they are being charged—a major issue preventing their practical use—is strongly and positively correlated with the degree of crystallinity of lithium peroxide (Li2O2), a compound produced during a discharge cycle. This discovery is expected to provide significant insight into resolving this issue.
- Because theoretical energy densities of lithium-air batteries are extremely high, they have the potential to be used in a wide variety of technologies, such as drones, IoT devices, electric vehicles and home electricity storage systems. The most serious issue concerning these batteries is the elevated voltage during a charge cycle (i.e., overpotential), which induce side reactions detrimental to their cycle lives. The causes of overpotentials had previously been largely unknown.
- To address this issue, this research team investigated the crystallinity of Li2O2—a product formed during a discharge cycle—and found that the Li2O2 with a more disordered crystalline structure (i.e., a low degree of crystallinity) is more susceptible to decomposition at a lower voltage during a charge cycle. We are the first to report this fact. Two types of discharge reaction pathways leading to the formation of Li2O2 have been known: reactions occurring on the surface of the carbon electrode and reactions occurring within the electrolyte (in the form of disproportional reactions). Our research found that the Li2O2 formed through pathway is decomposable at 3.5 V or lower voltage during a charge cycle while that formed through pathway is more resistant to decomposition, requiring 4 V or higher voltage. Furthermore, we found that the pathway Li2O2 had a more disordered crystalline structure than the pathway Li2O2 . These results indicate that the elevated charge voltage is induced by the Li2O2 with a highly ordered crystalline structure formed through pathway and that the high voltage can be lowered by suppressing the formation of this type of Li2O2.
- Based on these results, we will investigate ways to enable lithium-air batteries to primarily produce Li2O2 with a low degree of crystallinity, thereby significantly increasing their cycle lives and accelerating research efforts in putting them into practical use at the NIMS-SoftBank Advanced Technologies Development Center.
- This project was carried out by a research team led by Arghya Dutta (Postdoctoral Researcher, Center for Green Research on Energy and Environmental Materials (CGREEM), NIMS), Yoshimi Kubo (Advisor, NIMS-SoftBank Advanced Technologies Development Center; also a former leader of the Metal-Air Battery Sub Team, a part of the ALCA-SPRING Next-Generation Battery Team, until March 2020), Akihiro Nomura (Senior Researcher, CGREEM, NIMS) and Kimihiko Ito (Principal Researcher, Research Center for Advanced Measurement and Characterization, NIMS).
This project was mainly supported by the JST ALCA-SPRING program (ALCA: Advanced Low Carbon Technology Research and Development Program, SPRING: Specially Promoted Research for Innovative Next Generation Batteries).
This research was published in Advanced Science, an open access journal, on August 11, 2020, GMT.
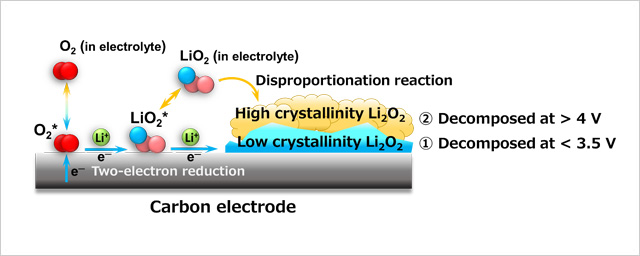
Figure. Schematic diagram illustrating the two Li2O2 formation pathways during a discharge cycle in a lithium-air battery
Related files
- Center for Green Research on Energy and Environmental Materials
- Softbank-NIMS Advanced Technologies Development Center
Contact information
(Regarding this research)
-
Yoshimi Kubo
Advisor,
Softbank-NIMS Advanced Technologies Development Center
National Institute for Materials Science
Tel: +8180-1292-2442
E-Mail: KUBO.Yoshimi=nims.go.jp
(Please change "=" to "@")
(General information)
-
Public Relations Office
National Institute for Materials Science
Tel: +81-29-859-2026
Fax: +81-29-859-2017
E-Mail: pressrelease=ml.nims.go.jp
(Please change "=" to "@")
(Regarding JST projects)
-
Masaru Oya
Department of R&D for Future Creation
Japan Science and Technology Agency
Tel; +81-3-3512-3543
E-Mail: alca=jst.go.jp
(Please change "=" to "@")
Same Keywords
-
Development of 1-Wh-Class Stacked Lithium-Air Cells
(lithium-air batteries,lithium)
2025.09.19
-
Chemical Crossover Accelerates Degradation of Lithium Electrode in High Energy Density Rechargeable Lithium–Oxygen Batteries
(lithium,SoftBank)
2023.01.31
-
3D Printing Nickel Single Crystals Using Laser Additive Manufacturing Technology
(crystal)
2022.06.22
Recent Press Release
-
No Need for Rare Earths or Liquid Helium! Cryogenic Cooling Material Composed Solely of Abundant Elements
2025.12.24
-
Novel Materials Design Approach Achieves a Giant Cooling Effect and Excellent Durability in Magnetic Refrigeration Materials
2025.12.19
-
Multiple Autonomous AI Systems Spontaneously Collaborate to Advance Materials Research
2025.12.10
