Finding the Relationship between the Structure and Catalytic Activity of Ruthenium Nanoparticles
—Achieved by Quantification of Local and Average Structures.
Accumulating Data for Machine Learning to Create New functional Materials—
2016.10.28
National Institute for Materials Science (NIMS)
Kyoto University
A research team consisting of NIMS and Kyoto University identified the possibility that slight differences in the structure of ruthenium (Ru) nanoparticles, which have high CO oxidation catalytic activity, may affect their catalytic functions. The team took the approach of quantifying both local atomic-scale structures and average structures.
(“Size dependence of structural parameters in fcc and hcp Ru nanoparticles, revealed by Rietveld refinement analysis of high-energy X-ray diffraction data”; Chulho Song, Osami Sakata, Loku Singgappulige Rosantha Kumara, Shinji Kohara, Anli Yang, Kohei Kusada, Hirokazu Kobayashi & Hiroshi Kitagawa; Scientific Reports 6, Article number: 31400 (2016) doi:10.1038/srep31400)
Abstract
- A research team led by Osami Sakata, Director of the Synchrotron X-ray Station at SPring-8, RNFS, NIMS, and Hiroshi Kitagawa, Professor of the Graduate School of Science, Kyoto University, identified the possibility that slight differences in the structure of ruthenium (Ru) nanoparticles, which have high CO oxidation catalytic activity, may affect their catalytic functions. The team took the approach of quantifying both local atomic-scale structures and average structures. Quantification of nanoparticle structures and accumulation of data on relationships between structures and functions of nanoparticles, as performed in this study, may further speed up the creation of new functional materials using data science methods such as machine learning.
- Some materials demonstrate superb functionality when they are processed into smaller pieces like nanoparticles. Previously, all Ru bulk materials had a hexagonal close packed (hcp) structure, but the research team succeeded in processing Ru materials into nanoparticles with a face-centered-cubic (fcc) structure. The team found earlier that fcc-type Ru nanoparticles have higher CO oxidation catalytic activity than conventional hcp-type Ru nanoparticles, but did not know why. Many nanoparticles have more or less periodic atomic arrangements. Since Ru nanoparticles consist of fewer atoms, it had been suggested that atoms that were displaced from their lattice sites possibly contribute to the difference in catalytic activity.
- To investigate this point, we studied local structures of Ru nanoparticles by focusing on a nearest neighboring atoms within a small area of a nanoparticle, and average structures across entire nanoparticles. Then we analyzed the relationship between these structures and catalytic activities of nanoparticles. Specifically, we studied the crystal structures of Ru nanoparticles in detail by measuring diffraction and scattering of high-energy X-rays at SPring-8. Nanoparticle structures were quantified using structural parameters in terms of a peak width of the first coordination shell as a function of interatomic distances and a width of the main peak in the bond angle distribution. From this quantification, we found that the structure of fcc-type nanoparticles is disorderly at a local level when particle sizes are around 3.5 nm to 5 nm. On the other hand, when hcp-type nanoparticles were observed, their structures were more orderly in terms of lattice distortion and average atomic positions above 3nm. Based on these results, we concluded that it is feasible to correlate local and average (lattice distortion and average atomic positions) structures of nanoparticles with catalytic activities of the nanoparticles.
- This study indicated that atomic configuration characteristics of nanoparticles, which had been thought to be irrelevant to nanoparticles’ catalytic activities and functions, may be in fact associated with catalytic activities. In the future, we plan to systematically study new functional materials at various nanoscales, and accumulate data on local and average structures of nanoparticles for use by machine learning. In so doing, we aim to set a foundation for implementing materials informatics to analyze the data.
- This research was supported by the Japan Science and Technology Agency (JST) through the ACCEL project “Creation of functional materials on the basis of the inter-element-fusion strategy and their innovative applications” (Professor Hiroshi Kitagawa, research director).
- This study was published in the 10-27-2016 issue of Physical Chemistry Chemical Physics. Also, a part of study complementary to this one was published in Scientific Reports on August 10, 2016.
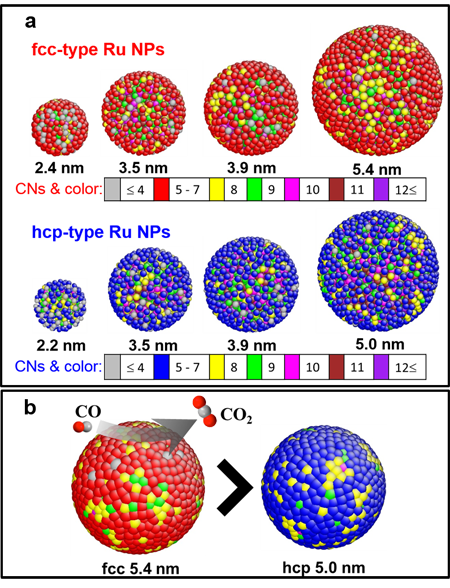
Figure. Three-dimensional atomic configurations of face-centered-cubic (fcc) -type and hexagonal close packed (hcp) -type ruthenium nanoparticles estimated by reverse Monte Carlo (RMC) modeling at the atomic scale. (a) Configurations of nanoparticles of different sizes. (b) CO conversion sites of a 5.4 nm fcc-type particle and a 5.0 nm hcp-type particle. Different colors represent different coordination numbers (CNs). For fcc-type particles, CNs increase in the order of red, yellow and green; for hcp-type particles, CNs increase in the order of blue, yellow and green.
Related files
- Synchrotron X-ray Station at SPring-8
Contacts
(Regarding this research)
-
Osami Sakata
Director of the Synchrotron X-ray Station at SPring-8; also a leader of the Synchrotron X-ray Group, Research Center for Advanced Measurement and Characterization, Research Network and Facility Services Division (RNFS), National Institute for Materials Science
Tel: +81-791-58-1970
E-Mail: SAKATA.Osami=nims.go.jp
(Please change "=" to "@")
(Regarding nanoparticle samples)
-
Hiroshi Kitagawa
Professor, Department of Chemistry, Graduate School of Science, Kyoto University
Tel : +81-75-753-4035
E-Mail: kitagawa=kuchem.kyoto-u.ac.jp
(Please change "=" to "@")
(For general inquiries)
-
Public Relations Office
National Institute for Materials Sciences
1-2-1 Sengen, Tsukuba, Ibaraki, 305-0047, Japan
Tel: +81-29-859-2026
Fax: +81-29-859-2017
E-Mail: pressrelease=ml.nims.go.jp
(Please change "=" to "@") -
International Public Communications Office, Public Relations Division, Planning and Information Management Department, Kyoto University
36-1, Yoshida-honmachi, Sakyo-ku, Kyoto, 606-8501, Japan
Tel: +81-75-753-5727
E-Mail: comms=mail2.adm.kyoto-u.ac.jp
(Please change "=" to "@")
Recent Press Release
-
Simultaneous Imaging of Intracellular DNA and RNA Using Harmless Light
2025.10.27
-
Development of an AI Device Using Ion Gel and Graphene That Dramatically Streamlines Machine Learning Computations
2025.10.14
-
Demonstrating a Novel Method to Modulate Heat Flow Through the Collective Motion of Spins
2025.10.06
