- Home
- > Outreach
- > Publications
- > CONVERGENCE
- > ASKING THE RESEARCHER
 ASKING THE RESEARCHER
ASKING THE RESEARCHER
Katsuhiko Ariga MANA Principal Investigator, Unit Director, Supermolecules Unit, Nano-Materials Field
The Future of Science, Perspective in Supermolecules
What is "supramolecular" chemistry?

"The ultimate materials may actually be the biological materials that have created us. Living organisms are autonomous and can also reproduce. They respond to stimuli from the outside world. The mechanism that makes biological activity possible is not the product of a serial arrangement of individual molecules; it's the result of judgments and responses by the total organism, in which various molecules are interrelated," says Dr. Ariga. For example, in the flagellar motors of bacteria, a machine-like system is created naturally when an extremely large number of protein molecules come together. The result of this process, in which molecules come together to form assemblies that manifest functions not possible in single molecules, is called a "supermolecule." "The lipids, proteins, and saccharides that make up living organisms are all combinations of many individual molecules and have the mechanism of supermolecules which can display feedback functions."
Dr. Ariga began full-scale research on supermolecules at the beginning of the 1990s, and he participated as Group Leader in the Japan Science and Technology Agency's International Cooperative Research Project (ICORP) [a1] "Supermolecules Project," which began in 1992. He also participated in the symposium "Supramolecular Chemistry: 100 Years of the Lock-and-Key Principle," which was held in Mainz, Germany in 1994. The main topic of that international symposium was the fact that "lock-and-key principle," which was proposed in connection with enzyme reactions, etc., grew into the concept of supermolecules over its history of almost 100 years, and was the subject of the Nobel Prize in 1987. Dr. Ariga says that he senses that a new current arose in supermolecules, with that 100-year anniversary as a turning point.
In actuality, following this, heightened expectations were placed on supermolecules in the life science and medical fields. The fact that supermolecules would play an important role in nanotechnology was also recognized. As a result, even greater attention was focused on supramolecular chemistry. Dr. Ariga became a key person at MANA and is leading the development of innovative supermolecule-based materials which are without precedent anywhere in the world.
"Technology that is useful" and "Science that pursues dreams."
Dr. Ariga says that he is "conscious of two concepts" in research. "One is 'technology that is useful' and the other is 'science that pursues dreams.' Living as a scientist who does research that only he is doing, that only he can do, and continuing that research based on free conception become a motivation."
As an example of Dr. Ariga's "technology that is useful," the development of a technology for visualizing radioactive cesium can be mentioned. After this research was announced in the media at the end of 2012, reagents were marketed by private companies within six months and reached practical application with exceptional speed. With radiation detectors and other physical techniques, it is not possible to see the presence of cesium visually, and there are limits to spatial resolution. In contrast, Dr. Ariga realized a technique for visualizing cesium with micrometer level resolution by designing a fluorescent probe, "Cesium Green," which is a supermolecule that distinguishes only cesium (Fig. 1).
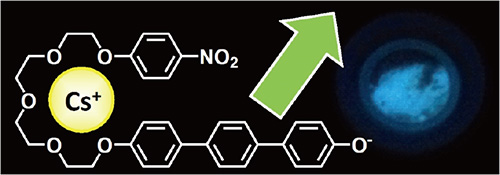
Fig. 1 Molecular structure of the cesium probe, "Cesium Green," for detection of cesium. When the supermolecule contains cesium, it emits a green light.
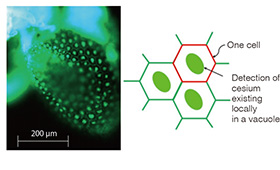
Fig. 2 Fluorescent image of a seed leaf of cress thale (Arabidopsis thaliana ) obtained by dripping a Cesium Green methanol solution. Bright fluorescent light can be observed from parts that are considered to be vacuoles in cells.

Fig. 3 Supramolecular system for grasping molecules with human hand.
On the other hand, the creation of "hand-operating nanotechnology" is an example of "science that pursues dreams." In 2010, Dr. Ariga developed a "Supermolecular system for grasping molecules with the human hand" (Fig. 3). This makes it possible to bend or stretch a molecular machine by pushing it with the human hand, and to capture and release different small molecules in water. This was realized by Dr. Ariga's original concept of reducing the movement of molecules from the 3-dimensional world to the 2-dimensional world. In other words, if molecules are arranged as a supramolecular thin film, and the spatial dimension in the height direction is brought infinitely close to zero, it is possible to couple large in-plane material-level movements and the molecular level action in the film direction. If the film is manually compressed from the side, the open molecular machine will try to take the smallest possible structure. As a result, it will assume a cavity structure, and in this process, it will capture molecules in the water (Fig. 4).
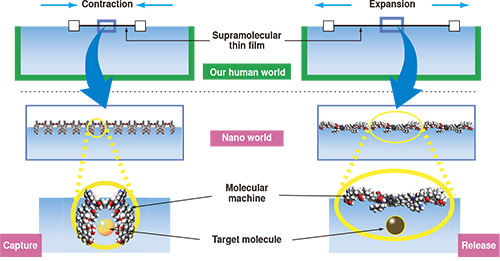
Fig. 4 Mechanism of the supramolecular system for grasping molecules with the human hand. When a supramolecular thin film on the surface of water is made to contract or expand by the movement of the human hand in our human world, corresponding to this, the molecular machine captures or releases a target molecule in the nano world.
It is also conceivable that the same phenomenon can also be realized not only in water, even by using a film that can be expanded and contracted freely, and Dr. Ariga is now developing a portable type system. The day when ordinary people can easily perform nanotech operations by hand, from sensing and capture of poisons to transmission of electrical signals via chemical substances, may not be far off!
To the science and technology of the future by "nanoarchitectonics"
In June of this year, Dr. Ariga published a book entitled "Materials Revolution: Nanoarchitectonics" for general readers (introduced on p. 11 of this issue of Convergence). As Dr. Ariga says, "Since the concept of nanotechnology is becoming stereotyped, here, we must achieve a breakthrough."
"We need to change our paradigm from the viewpoint of conventional nanotechnology, which considered the functions of individual nanoscale structures separately, to a total viewpoint that focuses on unknown functional linkages that are produced by accumulating interactions without knowing what will occur, which is due to the unique indeterminacy of the nano size. In our exploration of the nanoscale world, we have come to the time when we must aim at a change from simple technology, i.e., nanotechnology, to a total architecture, in other words, nanoarchitectonics." In this connection, it's worth mentioning the Dr. Ariga and Dr. Richard Feynman, who is the father of nanotechnology, share the same birthday.
Dr. Ariga also wants the more general public to understand how important science is. This is because he hopes that society will recognize not only science and technology that are immediately useful, but also, as an investment in the future, science that does not fear failure. Dr. Ariga believes that it is precisely this recognition of science by society that will nurture an environment in which scientists with new ideas can devote themselves wholeheartedly to their research. "Even if people call you foolish, follow your dream. And if you sometimes seem to be a heretic, pursue what only you can do. For Japanese science and technology to make further progress, we have to create a society where that's accepted."

