Careers paths at MANA : Focus on chirality
Jan Labuta
Independent Scientist, MANA
“My career as a research scientist in Japan started in November 2008 when I joined the Supramolecules Group at MANA for a two‐year JSPS postdoctoral fellowship,” says Labuta. “I was really impressed and inspired by the highly focused and passionate approach to research at MANA. I learnt a lot and was able to contribute to the research activities of the group. It was a very fruitful two years for me and the experience led me to pursue a long term career at MANA. I highly recommend this career path to other young researchers overseas.”
In 2014 Labuta was selected as a ICYS‐Sengen fellow as part of the highly competitive International Center for Young Scientists (ICYS) program [1]. ICYS fellows work with mentors and using their funding from NIMS to pursue innovative research at the facilities offered by MANA and NIMS. Furthermore, MANA provides full English language clerical support to enable members of the ICYS program to concentrate on their research and not administrative issues.
“The ICYS program enabled me to build on my previous research,” says Labuta. “With advice and support from my mentor I also learnt how to manage research within this large organization. Now, as an independent scientist [2] at MANA , I am my own boss. I have complete freedom to conduct my research. It is a busy life with funding applications, recruitment of young researchers, and of course family life!”
Collaborative research: Bridge between alma mater and MANA
“Some of my group members, mostly Ph.D. students, are from Charles University, Prague, my alma mater,” says Labuta. “I regularly invite researchers from there to work with me at MANA and I use their NMR and MRI facilities. Such openness to international collaboration is one of the strengths of being an independent scientist at MANA. In addition to the research visitors also have an opportunity to experience Japanese culture at first hand. And make many connections for the future.”
Research activities
Jan Labuta’s research is focused on organic dyes called porphyrins, contained in, for example, hemoglobin, chlorophyll, and vitamin B12. One of the goals is to use porphyrins as sensors to detect the chirality of molecules—that is right and left handedness of compounds, where their mirror images cannot be superimposed on top of each other.
Importantly, knowledge of the chiral properties of molecules is important for the development of pharmaceuticals because their biological activity in the body depends on the identity of the enantiomer, namely, whether it is left or right handed. Pharmaceuticals having the same chemical structure but with different chirality can cause severe side effects when administered to treat ailments.
“Approximately 80% of drugs approved by the FDA are chiral and 75% are single enantiomers,” explains Labuta. “For example left handed thalidomide is a sedative and safe, but the right handed compound is teratogenic and harmful to health. So it is critical to monitor the purity of chiral compounds.”
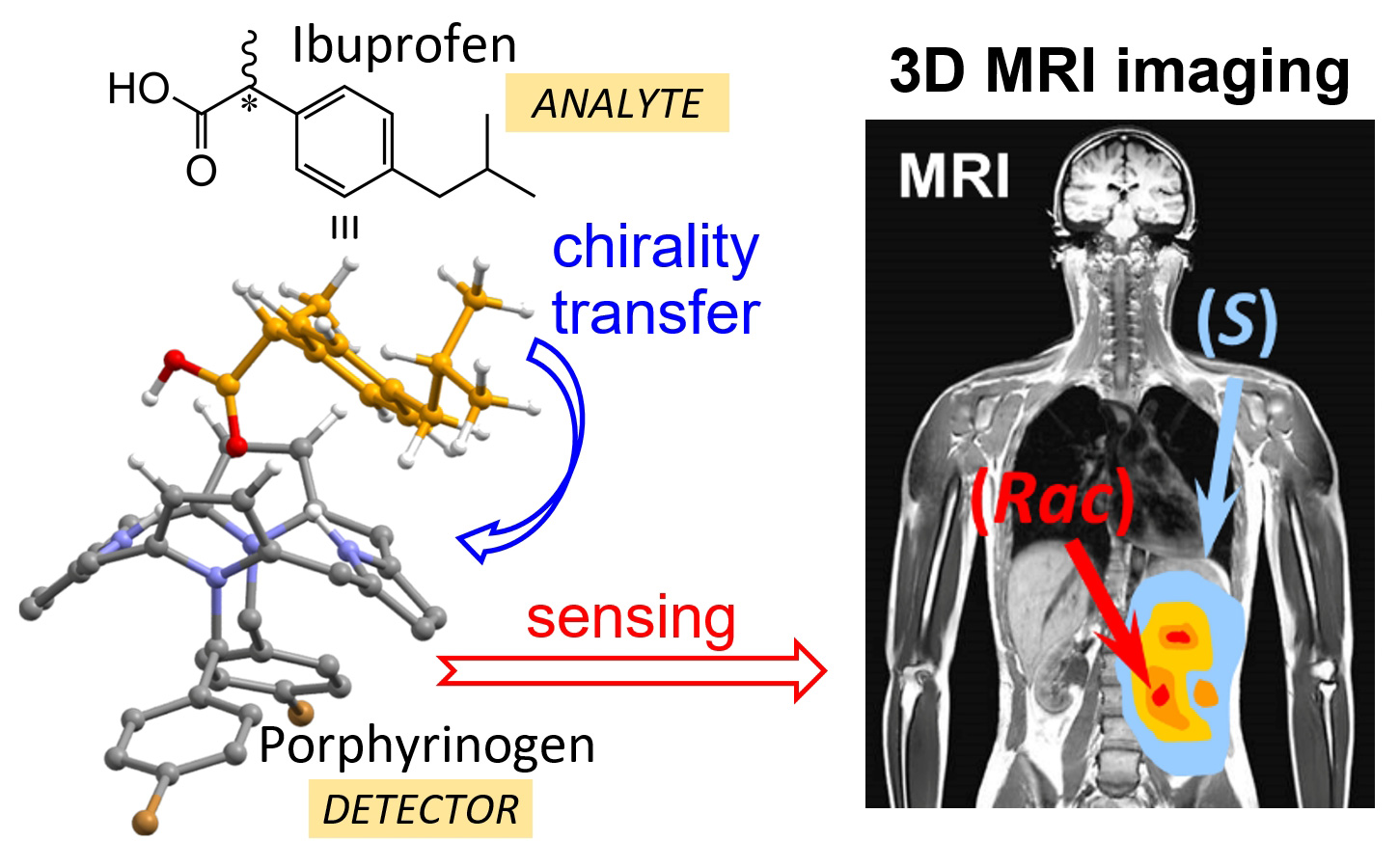
Schematic visualization of MRI in vivo chirality mapping of chiral analyte using porphyrintype molecule as a detecting agent.
Labuta and his colleagues are devising methods to monitor and image chirality of molecules in situ or in vivo, which can be useful for the development and analysis of new drugs. In their approach they synthesize customized detector molecules (mainly porphyrins) and analyze their sensing properties using nuclear magnetic resonance (NMR).
Recently, they have a developed method for detecting chirality by NMR where the information about the chirality is transferred to achiral detector molecules (porphyrin) and subsequently read out using NMR. With further improvements this method shows great potential for determining chirality.
“Our ultimate goal is to produce a method for 3D spatial and temporal MRI monitoring of chirality in real‐time during chemical reactions and inside living systems.”
#####

Reference / Media / Publications
[1]
International Center for Young Scientist (ICYS)
https://www.nims.go.jp/eng/hr-development/icys/
https://www.nims.go.jp/icys/
[2]
“MANA Independent Scientists System: Total freedom to conduct your own research”, MANA eBulletin Feature article, Yoshio Bando, Akihiro Okamoto & Gaku Imamura, Vol.3, June 2018
https://www.nims.go.jp/mana/ebulletin/feature_03.html
[3]
“NMR Spectroscopic Determination of Enantiomeric Excess Using Small Prochiral Molecules.” S. Ishihara, J. Labuta, Z. Futera, S. Mori, H. Sato, K. Ariga and J. P. Hill, J. Phys. Chem. B, 122, 5114–5120 (2018).
[4]
“Chiral Sensing by Nonchiral Tetrapyrroles.” J. Labuta, J. P. Hill, S. Ishihara, L.Hanyková, K. Ariga, Acc. Chem. Res., 48, 521–529 (2015).
[5]
“Chiral Guest Binding as a Probe of Macrocycle Dynamics and Tautomerism in a Conjugated Tetrapyrrole.” J. Labuta, Z. Futera, S. Ishihara, H. Kouřilová, Y. Tateyama, K. Ariga and J. P. Hill, J. Am. Chem. Soc., 136, 2112–2118 (2014).
[6]
“NMR Spectroscopic Detection of Chirality and Enantiopurity in Referenced Systems without Formation of Diastereomers.” J. Labuta, S. Ishihara, T. Šikorský, Z. Futera, A. Shundo, L. Hanyková, J. V. Burda, K. Ariga and J. P. Hill, Nature Communications, 4, 2188 (2013).
