Novel functional molecular liquids developed by alkyl-π engineering
T. Nakanishi
We developed novel ultimate-soft organic materials, i.e., room-temperature functional molecular liquids composed of aπ-conjugated molecular unit bearing bulky, flexible branched alkyl chains. The studies of full-color tunable luminescent liquids and uncommon phase phenomena with the photoconducting property of liquid fullerenes are designed simply by controlling a balance of intermolecular interactions in the alkyl-π compounds, i.e., van der Waals and π–π interactions among adjacent molecules, or “alkyl-π engineering”.
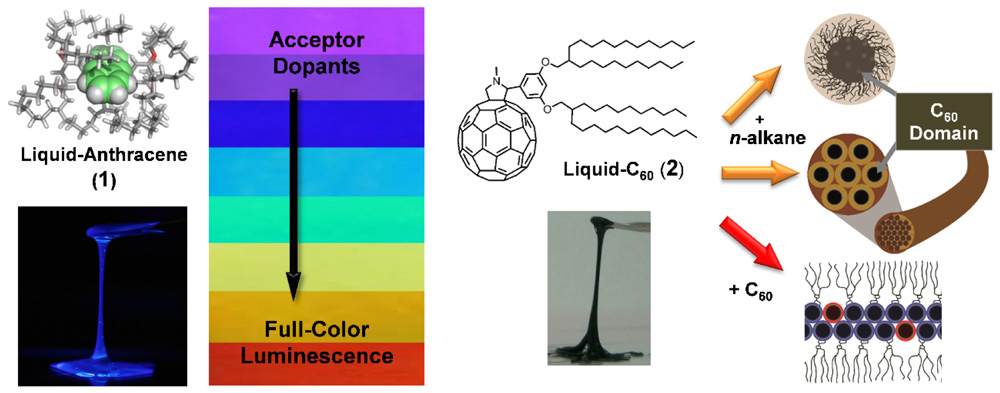
Apart from ordered fluid materials, i.e., liquid crystals, solvent-free room-temperature functional molecular liquids (FMLs)[1] attract attention as novel ultimate-soft organic materials. FMLs possess versatile processability, like geometry-independent coating and filling into narrow spaces via capillary action, and excellent stability under heat as well as great deformability. In addition, as a solvent function FMLs can accommodate other functional molecules within them and allow the adjustment of their properties in a predictable manner. Thus, FMLs can overcome many issues encountered in organic/polymeric substances for their practical use. Here, the molecular design principle of FMLs based on an alkylated-π molecular system as well as their luminescence and optoelectronic properties are introduced.
Our first molecular design was a luminescent π-conjugated core isolated by the attached bulky, flexible branched-alkyl chains, and the selected core is a blue-luminescent anthracene ((1), Fig. 1)[2]. Using this strategy, we achieved an intrinsic molecular optical property, i.e., luminescence, even in the solvent-free neat state showing almost the same optical features as a dilute solution. The liquid character of (1) allows for accommodation of energy-accepting emissive dopants, which inspires the creation of full-color luminescent liquid (Fig. 1(a)). In addition, the anthracene core is effectively wrapped with the bulky side chains, thus preventing its photodegradation.
Another research strategy is to direct the assembly of room-temperature liquid alkyl-C60 molecules (e.g., (2), Fig. 1). The C60 unit substituted with two branched alkyl chains on one side has an asymmetric molecular structure. Using additives that favor either the alkyl (n-alkane) or π-conjugated (pristine C60) part, amorphous alkylated-C60 materials are assembled into ordered structures such as micelles and gel fibers with n-alkanes, and lamellar nanosheets with C60 (Fig. 1(b))[3]. The resulting gel fibers and lamellar nanosheets are composed of well-organized C60 moieties, thus changing them to photoconductive from the insulating disordered liquid state.
Main Papers
- “Nonvolatile functional molecular liquids”, S.S. Babu, T. Nakanishi, Chem. Commun. 49 (2013) 9373 (feature article, front cover picture).
- “Nonvolatile liquid anthracenes for facile full-colour luminescence tuning at single blue-light excitation”, S.S. Babu, M.J. Hollamby, J. Aimi, H. Ozawa, A. Saeki, S. Seki, K. Kobayashi, K. Hagiwara, M. Yoshizawa, H. Möhwald, T. Nakanishi, Nat. Commun. 4 (2013) 1969.
- “Directed assembly of optoelectronically active alkyl-π-conjugated molecules by adding n-alkanes or π-conjugated species”, M.J. Hollamby, M. Karny, P.H.H. Bomans, N.A.J.M. Sommerdijk, A. Saeki, S. Seki, H. Minamikawa, I. Grillo, B.R. Pauw, P. Brown, J. Eastoe, H. Möhwald, T. Nakanishi, Nat. Chem. 6 (2014) 690.