Chiral sensing: novel chiral solvating agents for nondiastereomeric determination of enantiomeric excess
J. Labuta , S. Ishihara , K. Ariga , J. P. Hill
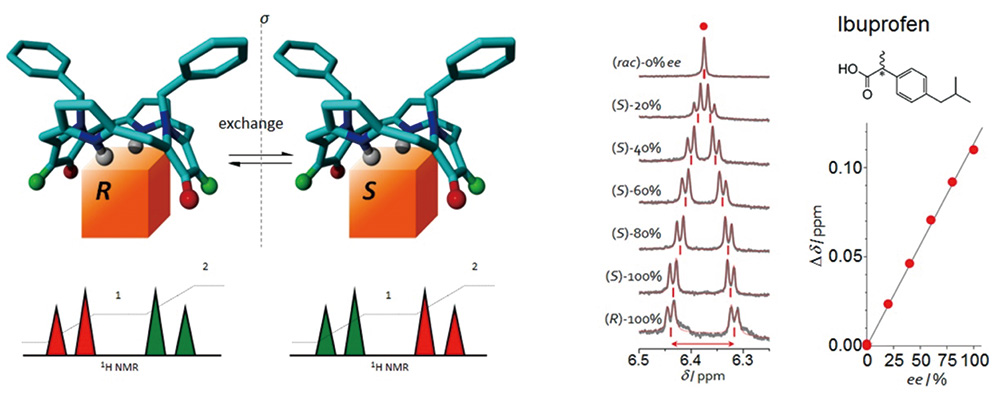
We developed a unique family of prochiral chiral solvating agents (pro-CSA) for the determination of enantiomeric excesses (ee) of a wide range of chiral analytes including carboxylic acids, alcohols, amines, and ketones using nuclear magnetic resonance (NMR) spectroscopy. This is made possible by the weak interaction and consequent rapid solution exchange between analyte and pro-CSA and can be considered as the sampling of the average chirality of the analyte in solution.
Chiral parameters of asymmetric compounds, including their absolute configurations and enantiomeric purity, are important in medical and pharmaceutical applications. Our achiral molecular system allows for the rapid analysis of enantiomeric purity in a wide range of organic substances, which not only facilitates the development of chiral drugs but also permits the study of asymmetric reaction pathways and chiral drug metabolism. These are important from the point of view of the widely used chiral catalysts and also suggest the use of our reagents for monitoring dynamic chiral transformations. Being achiral, our reagents are ideal for such purposes since they would not affect the outcome of such reactions in terms of their chirality.
ee can be determined by constructing a calibration curve where ee is proportional to a splitting in selected peaks in the NMR spectrum of the analyte[1][2]. This procedure allows for the rapid analysis of ee in, for instance, a pharmaceutical development scenario, where time-consuming high-performance liquid chromatography can be replaced by the commonly used NMR spectroscopy. In particular, the use of our pro-CSA should improve optimization times for asymmetric reaction development. Also, when a symmetrical molecule is adapted as an ee sensor, it has certain intrinsic advantages such as identical binding constants for each enantiomer, resulting in ee determination that is not obscured by kinetic resolution[2]. Our system can also be used to improve our understanding of important chirality principles such as majority rule and intermolecular chirality transfer. As a result of our careful molecular design, pro-CSA systems based on nanometric saddle-shaped tetrapyrroles for room-temperature NMR determination of ee values in a wide range of analyte types including acids, esters, amines (including amino acid derivatives), and ketones were established[3].
Main Papers
- “Nuclear magnetic resonance signaling of molecular chiral information using an achiral reagent”, A. Shundo, J. Labuta, J.P. Hill, S. Ishihara, K. Ariga, J. Am. Chem. Soc. 131 (2009) 9494.
- “NMR spectroscopic detection of chirality and enantiopurity in referenced systems without formation of diastereomers”, J. Labuta, S. Ishihara, T. Šikorský, Z. Futera, A. Shundo, L. Hanyková, J.V. Burda, K. Ariga, J.P. Hill, Nat. Commun. 4 (2013) 3188.
- “Chiral sensing using non-chiral porphyrins”, J. Labuta, J.P. Hill, S. Ishihara, L. Hanykova, K. Ariga, Acc. Chem. Res. 48 (2015) 521.