Sep. 25, 2025
Report an inexpensive iron hydroxide catalyst that could support the use of sodium borohydride as a hydrogen storage material.
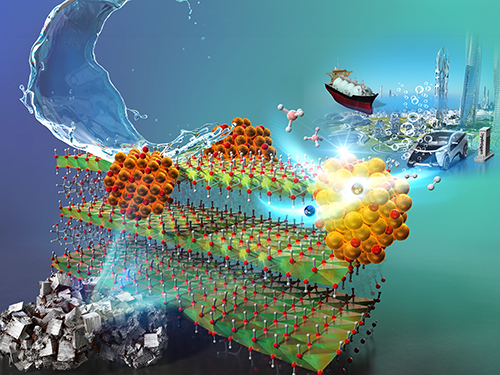
As the world moves toward hydrogen-powered societies, one major challenge remains: storing and releasing hydrogen efficiently. Sodium borohydride (SBH) is a promising hydrogen storage material that can generate hydrogen through simple contact with water. However, this reaction typically relies on expensive catalysts made from precious metals like platinum, limiting its large-scale use.
In a recent breakthrough, researchers from the Layered Nanochemistry Group at MANA, led by group leader Dr. Yusuke Ide, along with Mr. Ezz-Elregal M. Ezz-Elregal and Dr. Mitsutake Oshikiri, developed a cost-effective, high-performance catalyst using ‘green rust’— a mixed-valent iron hydroxide mineral once considered too unstable for practical use.
The key lies in modifying green rust particles with a copper chloride solution. This process forms nanoscale copper oxide clusters at the particle’s edges, generating highly active sites for hydrogen production. The green rust structure also absorbs sunlight, transferring energy through the copper clusters to boost the reaction’s efficiency even further.
Performance tests revealed that the new catalyst achieves a high turnover frequency for hydrogen production comparable to or even exceeding those of traditional precious metal-based materials. It also showed excellent durability, maintaining catalytic activity through repeated use.
What makes this breakthrough especially promising is its scalability and practicality. The catalyst works at room temperature, is relatively easy to produce, and could integrate well with existing SBH-based hydrogen systems. With low-cost SBH production already being developed and pilot projects using the technology in hydrogen-powered ships, this advance could accelerate the global shift to clean hydrogen energy.
“We expect that our catalyst will be used for hydrogen fuel cells in many onboard applications like cars and ships,” says Dr. Ide, “This will hopefully lead to various forms of emission-free mobility.”
References
| Journal | ACS Catalysis |
|---|---|
| Title | A Catalyst for Sodium Borohydride Dehydrogenation Based on a Mixed-Valent Iron Hydroxide Platform |
| Authors | Ezz-Elregal M. Ezz-Elregal1,2,3, Koichi Shinohara4, Hamza El-Hosainy1,5, Takumi Miyakage6, Takashi Toyao6, Ken-ichi Shimizu6, Akio Iwanade7, Makoto Oishi7, Takuro Nagai7, Naoki Fukata1,8, Takumi Tsushima9, Hiroto Yoshida9, Mitsutake Oshikiri1, Yusuke Ide1,2 |
| Affiliations |
|
| DOI | 10.1021/acscatal.5c01894 |