- Home
- > Research
- > Hot Topics
 Hot Topics
Hot Topics
Introduction of "Development of Method to Identify Single π-Stacked Dimer of Naphthalene Molecules" by Kazuhito Tsukagoshi Group Leader, and a collaborative research team from Tokyo Institute of Technology
Development of Method to Identify Single π-Stacked Dimer of
Naphthalene Molecules
- Expected to contribute to the elucidation of mechanisms for pathological diagnosis and drug discovery -

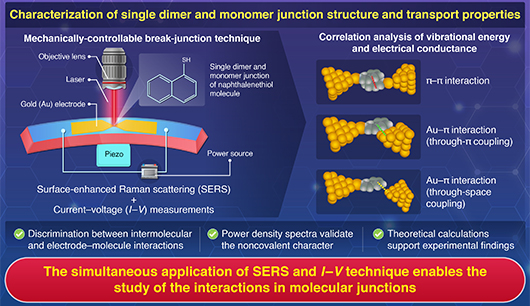
Kazuhito Tsukagoshi, Group Leader of the Thin Film Electronics Group and a collaborative research team from School of Science Department of Chemistry at Tokyo Institute of Technology have successfully identified a single dimer※1 of naphthalene molecules in a nano-gap structure.
The interactions mediated by π-electrons (π-π interactions※2) are well-known as crucial interactions that play a role in the bonding of crystals used in organic semiconductors, and in the binding of small molecules with proteins and DNA. In recent years, particular attention has been paid to π-stacked dimers, where molecules are connected via π-electrons. Because the electron transport properties of π-stacked dimers can change drastically with slight changes in the overlap of the π-orbitals, it is thought that elucidating this structure can pave the way for the development of new electronic materials, the elucidation of drug discovery mechanisms, and applications for pathological diagnoses. However, there has been no method to experimentally clarify the connection structure between electrodes in a dimer composed of single molecules, making it impossible to identify the state of interactions between molecules, and between metals and molecules.
In this study, a nano-gap structure was fabricated using microfabrication technology to break gold electrodes and trap molecules, aiming to recognize the connection structure of the dimer between electrodes. Using these gold electrodes, they simultaneously conducted "electrical measurements to detect current signals" and "Raman scattering measurements by laser light irradiation", and attempted to identify the molecular state by analyzing the current signals and vibration spectra of the molecules on the electrodes. As a result, they detected a vibration spectrum characteristic of naphthalene molecules and found a correlation between peaks derived from vibrations and current value fluctuations. From the correlation diagram for electrical conductivity and breathing vibration※3 energy, three types of states were detected. When analyzed using quantum chemical calculations, these three states were identified as, 1) a state where the π-electrons of naphthalene strongly interact with the electrode metal by chemically adsorb※4, 2) a state where naphthalene forms a dimer due to π-π interactions, and 3) a state where naphthalene weakly interacts with the electrode metal by physically adsorbs※5. This result indicates that they have successfully identified a single dimer of naphthalene molecules using a nano-gap structure.
The method developed by the collaborative research team enables the detection of dimerization of single molecules in environments where various devices operate in room temperature atmospheres. Furthermore, this method can be applied to a variety of functional molecules. Because electron transport via π-π interactions is not only important in many electronic materials but also deeply related to the recognition of biomolecules, it is expected to be applied to the development of minute electronic devices, contributions to the field of drug discovery, and the development of medical diagnostic methods for detecting pathological sites with high sensitivity in the future.
This collaborative research was conducted as part of the"Japan Society for the Promotion of Science, Grants-in-Aid for Scientific Research", "Tokyo Institute of Technology, ASUNARO Grant", "Tokyo Institute of Technology, Tokyo Tech Challenging Research Award".
※1 Dimer
A state where two molecules form a unit structure through mutual interaction.
※2 π-π Interaction
An interaction is caused by π-electrons in aromatic molecules such as benzene. It plays a role in the mechanism by which various molecules express their functions, such as the structure of molecular crystals and the binding of small molecules and proteins.
※3 Breathing Vibration
Vibration is where the skeleton of a molecule with a ring structure, like benzene or naphthalene, changes symmetrically.
※4 Chemical Adsorption
A state where the target substance and the molecule form a chemical bond through a chemical reaction.
※5 Physical Adsorption
A state where the target substance and the molecule are connected by intermolecular interactions.
The interactions mediated by π-electrons (π-π interactions※2) are well-known as crucial interactions that play a role in the bonding of crystals used in organic semiconductors, and in the binding of small molecules with proteins and DNA. In recent years, particular attention has been paid to π-stacked dimers, where molecules are connected via π-electrons. Because the electron transport properties of π-stacked dimers can change drastically with slight changes in the overlap of the π-orbitals, it is thought that elucidating this structure can pave the way for the development of new electronic materials, the elucidation of drug discovery mechanisms, and applications for pathological diagnoses. However, there has been no method to experimentally clarify the connection structure between electrodes in a dimer composed of single molecules, making it impossible to identify the state of interactions between molecules, and between metals and molecules.
In this study, a nano-gap structure was fabricated using microfabrication technology to break gold electrodes and trap molecules, aiming to recognize the connection structure of the dimer between electrodes. Using these gold electrodes, they simultaneously conducted "electrical measurements to detect current signals" and "Raman scattering measurements by laser light irradiation", and attempted to identify the molecular state by analyzing the current signals and vibration spectra of the molecules on the electrodes. As a result, they detected a vibration spectrum characteristic of naphthalene molecules and found a correlation between peaks derived from vibrations and current value fluctuations. From the correlation diagram for electrical conductivity and breathing vibration※3 energy, three types of states were detected. When analyzed using quantum chemical calculations, these three states were identified as, 1) a state where the π-electrons of naphthalene strongly interact with the electrode metal by chemically adsorb※4, 2) a state where naphthalene forms a dimer due to π-π interactions, and 3) a state where naphthalene weakly interacts with the electrode metal by physically adsorbs※5. This result indicates that they have successfully identified a single dimer of naphthalene molecules using a nano-gap structure.
The method developed by the collaborative research team enables the detection of dimerization of single molecules in environments where various devices operate in room temperature atmospheres. Furthermore, this method can be applied to a variety of functional molecules. Because electron transport via π-π interactions is not only important in many electronic materials but also deeply related to the recognition of biomolecules, it is expected to be applied to the development of minute electronic devices, contributions to the field of drug discovery, and the development of medical diagnostic methods for detecting pathological sites with high sensitivity in the future.
This collaborative research was conducted as part of the"Japan Society for the Promotion of Science, Grants-in-Aid for Scientific Research", "Tokyo Institute of Technology, ASUNARO Grant", "Tokyo Institute of Technology, Tokyo Tech Challenging Research Award".
※1 Dimer
A state where two molecules form a unit structure through mutual interaction.
※2 π-π Interaction
An interaction is caused by π-electrons in aromatic molecules such as benzene. It plays a role in the mechanism by which various molecules express their functions, such as the structure of molecular crystals and the binding of small molecules and proteins.
※3 Breathing Vibration
Vibration is where the skeleton of a molecule with a ring structure, like benzene or naphthalene, changes symmetrically.
※4 Chemical Adsorption
A state where the target substance and the molecule form a chemical bond through a chemical reaction.
※5 Physical Adsorption
A state where the target substance and the molecule are connected by intermolecular interactions.
Correlation diagram of the vibration energy and electrical conductivity of naphthalene (left) and states of interactions corresponding to each region in the diagram (right) (Source: Tokyo Institute of Technology)
Paper details
| Title | "Intermolecular and Electrode-molecule Bonding in a Single Dimer Junction of Naphthalenethiol as Revealed by Surface-enhanced Raman Scattering Combined with Transport Measurements" |
| Authors | Kanji Homma, Satoshi Kaneko, Kazuhito Tsukagoshi, Tomoaki Nishino |
| Journal | Journal of the American Chemical Society |
| DOI | 10.1021/jacs.3c02050 |
Contact information
Research Center for Materials Nanoarchitectonics (MANA)
National Institute for Materials Science
1-1 Namiki, Tsukuba, Ibaraki 305-0044 Japan
Phone: +81-29-860-4710
E-mail: mana-pr[AT]ml.nims.go.jp
1-1 Namiki, Tsukuba, Ibaraki 305-0044 Japan
Phone: +81-29-860-4710
E-mail: mana-pr[AT]ml.nims.go.jp

