Development of a Surgical Sealant Capable of Preventing Air Leaks in Inflated Lungs
—Chemically Modified Alaska Pollock Gelatin Proven to be Effective in Sealing Isolated Porcine Lungs, Ready for Preclinical Evaluation—
National Institute for Materials Science
University of Tsukuba
A NIMS-University of Tsukuba research group has developed a sealant for use in closing pulmonary surface wounds resulting from surgical resectioning. Highly conformable and resistant to pressure, this sealant—a chemically modified Alaska pollock-derived gelatin—effectively sealed wounds on the surface of porcine lungs, which remained free of air leaks after inflation. This sealant, a promising alternative to the human blood-derived fibrin sealants currently in common use, is scheduled for preclinical application.
( “Novel Alaska Pollock Gelatin Sealant Shows High Adhesive Quality and Conformability” Masatoshi Yamaoka, Naoki Maki, Ashoka Wijesinghe, Shoko Sato, Takahiro Yanagihara, Naohiro Kobayashi, Shinji Kikuchi, Yukinobu Goto,
Tetsushi Taguchi, Yukio Sato; journal : Annals of Thoracic Surgery; DOI:
10.1016/j.athoracsur.2018.11.074)
Abstract
- A NIMS-University of Tsukuba research group has developed a sealant for use in closing pulmonary surface wounds resulting from surgical resectioning. Highly conformable and resistant to pressure, this sealant—a chemically modified Alaska pollock-derived gelatin—effectively sealed wounds on the surface of porcine lungs, which remained free of air leaks after inflation. This sealant, a promising alternative to the human blood-derived fibrin sealants currently in common use, is scheduled for preclinical application.
- Air leaks in pulmonary surface wounds resulting from surgical resectioning for lung cancer treatment and other causes cannot be completely sealed by sutures. Fibrin sealant derived from human blood is currently used to close these wounds. Although fibrin sealant is highly compatible with biological systems due to its origin, it is only weakly adhesive to biological tissues and organs and is inadequately conformable to expansion and contraction of the lungs.
- To resolve these issues, this research group developed a sealant for respiratory surgery composed of Alaska pollock-derived gelatin modified with hydrophobic decanoyl groups that are highly adhesive to biological tissues and a polyethylene glycol-based crosslinker with proven clinical performance. The sealant was applied to 900 mm2 areas on the surfaces of isolated porcine lungs which were then inflated. During inflation, the sealant remained attached to the surface until the surface area increased by 2.9 times and demonstrated approximately twice the conformability of fibrin sealant, indicating that the sealant is sufficiently adhesive to remain on the surface of expanding and contracting lungs after surgery. In addition, the research group created and applied the sealant to holes 10 mm in diameter in isolated porcine lungs. The sealant demonstrated strong resistance to pressure, measuring 52.3 cm H2O—approximately 1.4 times that of fibrin sealant (37.5 cm H2O). These values indicate that the sealant would be capable of withstanding increased airway pressure caused by coughing. The sealant also hardens within five seconds of being applied to biological tissues and is biodegradable and absorbable by the body after the recovery of the treated tissues.
- In future studies, the NIMS-University of Tsukuba research group intends to conduct preclinical evaluation of the sealant with the goal of demonstrating proof of concept under the medicine-engineering collaborative research framework.
- This research project was carried out by a research group led by Tetsushi Taguchi (Group Leader, Biomaterials Field, Research Center for Functional Materials, NIMS) and Yukio Sato (Professor, Faculty of Medicine, University of Tsukuba). This project (location: University of Tsukuba, principal investigator: Tetsushi Taguchi) has been supported by the “Project for translational research program, Strategic promotion for practical application of innovative medical technology” sponsored by the Japan Agency for Medical Research and Development (AMED).
- This study was published in the online version of The Annals of Thoracic Surgery medical journal on February 18, 2019, local time and presented at the AMED annual meeting on February 27, 2019.
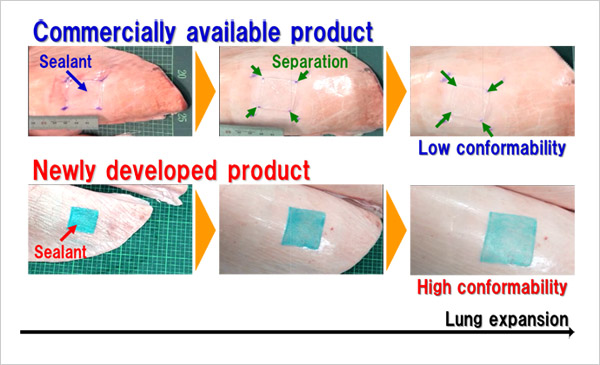
figure: Comparison of sealant