Four Research Fields

Effective Conversion of Materials and Energy is Crucial
to the Realization of a Sustainable Society
The “Nano-Power” field employs the concepts of surface nanoarchitectonics to directly control the nano structure on the atomic and molecular level. The research aims at illuminating and exploring methods and processes for interconversion between energy and matter with high efficiency.
Capturing the energy of the sun
Based on the concept of nanoarchitectonics, research in this area focuses on two approaches. One involves the use of semiconductor photocatalysts to produce hydrogen by splitting water, or to reductively fix carbon dioxide. The other approach involves semiconductors or metals with a layer of functional molecules on the surface for photoelectric conversion or for reductive fixation of carbon dioxide.
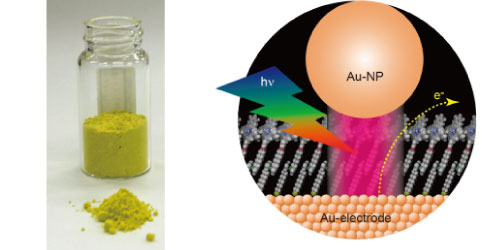
Left: Highly efficient photocatalyst for water oxidation responsive to visible light
Right: Enhancing photoelectric conversion efficiency through the use of nano gap light antenna effect
Efficient storage and use of energy
Secondary (rechargeable) batteries are the most widely used energy storage device, but they still leave a lot to be solved in terms of efficiency and safety. One way to solve such problems and drastically improve reliability is to turn the entire battery into a solid through the use of solid ionic conductors. If hydrogen is to be used as energy medium, the development of fuel cells that can convert hydrogen into electricity is essential. In both cases, the key to improve performance lies in increasing the conductivity of the ionic conductor and controlling interfacial structure. Towards this end, new electrolyte materials are being developed based on nanoarchitectonics and interfacial ion transfer mechanism is under investigation.
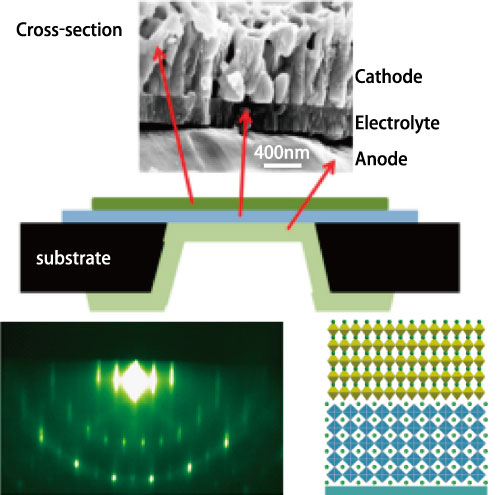
Top: Solid oxide fuel cell
Bottom: Highly oriented electrolyte for lithium ion battery
Programmed arrangement of atoms and molecules to form ultimate catalysts
To control and efficiently proceed chemical reactions, catalysts that accelerate each elemental step such as cleavage and formation of chemical bonds are essential. For complex chemical reactions, multi-functional catalysts are required. Ultimate catalysts to realize highest efficiency are being developed by utilizing surface nanoarchitectonics to arrange various atoms in desired positions.
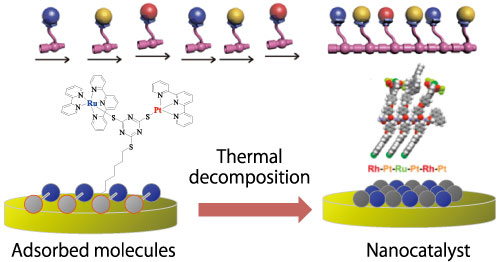
Controlled arrangement of molecules and conversion to highly efficient catalyst
Determination of Interfacial structure with spatial resolution of 1 billionth of a meter and time resolution of 10 trillionth of a second
In situ, real time determination of the structure of interfaces, particularly solid/liquid interfaces, at high spatial and temporal resolution is essential to achieve the efficient harvesting of solar energy, storage and usage of chemical energy, and catalyst reactions based on surface nanoarchitectonics. For this purpose, scanning probe microscopy, laser spectroscopy, x-ray scattering/absorption using synchrotron radiation and other advanced measurement techniques are developed to analyze interfacial structures with a spatial resolution to 1 billionth of a meter (1 nm) and measure electron transfer dynamics with a time resolution to 10 trillionth of a second (0.1 psec).
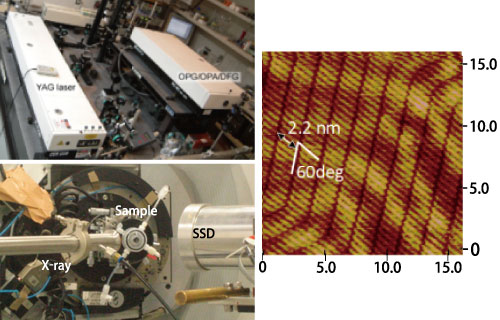
Top: Laser spectroscopy setup
Middle: STM image of molecule resolution in liquid
Bottom: X-ray absorption spectroscopy setup