Group
電子機能材料グループ Electronic Functional Materials Group
Result H22
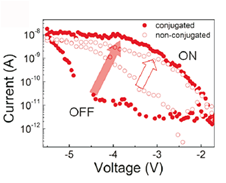
Tuning of Nonvolatile Bipolar Memristive Switching in Co(III) Polymer with an Extended Azo Aromatic Ligand,
A. Bandyopadhyay, S. Sahu and M. Higuchi, J. Am. Chem. Soc. 2011, 133, 1168–1171.
We have fabricated a unique memristive device by molecular engineering and demonstrated that the leakage current tuning in the device is 100 times more efficient than that in a standard device. Molecular analogs of the memristive matrices used here are an electrochemically active conjugated Co(III) polymer (CP) and a nonconjugated Co(III) polymer (NCP), which have been synthesized in good yield and characterized by 1H NMR spectroscopy. Redox switching of an organic-metallic hybrid polymer generates bistable states with a large ON/OFF ratio that supports random flip-flops for several hours. Thus, we provide a synthetic solution to leakage current restriction, one of the fundamental problems faced when fabricating state-of-the-art electronic devices.
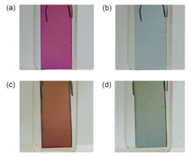
Electrochromic Properties of Polythiophene Polyrotaxane Film,
T. Ikeda and M. Higuchi, Langmuir 2011, 27, 4184–4189.
The electrochromic properties of a polythiophene polyrotaxane film consisting of a polythiophene backbone wrapped by the tetra-cationic cyclophane, cyclobis(paraquat-p-phenylene), were characterized. A naked reference polythiophene film, i.e., is the polythiophene without tetra-cationic cyclophane, was also characterized. The surface morphology was observed by atomic force microscopy. The surface of the naked reference polythiophene film has micrometer-scale polythiophene aggregates, which causes the dark color of the film. The polythiophene polyrotaxane gives a more homogeneous and brighter colored film owing to the suppression of molecular interactions between the polythiophene chains by the tetra-cationic cyclophanes. Potential-step chronoamperometric measurement provided the apparent diffusion coefficient of the charge transport in the film. Interestingly, the polythiophene polyrotaxane film afforded a significantly larger diffusion coefficient than the naked reference polythiophene film. This result suggests that the rate determining step of the charge transport is not the electron hopping between the polythiophene chains but the transport of charge-compensating counter ions from the solvent into the polythiophene. We believe that the counter anions of the tetra-cationic cyclophane provide a pathway allowing the charge-compensating counter anions to migrate from the solvent to polythiophene. The polythiophene polyrotaxane film showed faster color change than the naked reference polythiophene film in the electrochromic reaction. These results indicate that our polythiophene polyrotaxane is a better electrochromic material than the naked reference polythiophene.
