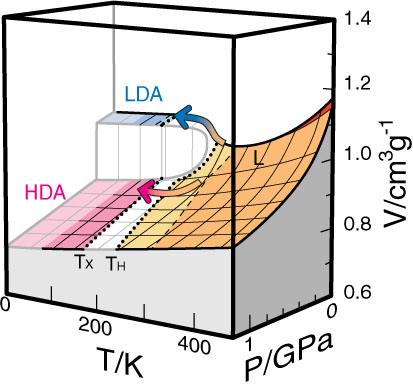
| 50. |
Transformation process of ice crystallized from a glassy dilute trehalose aqueous solution.
| Y. Suzuki & S. Takeya |
Phys. Chem. Chem. Phys., 24, 26659-26667 (2022). |
| 49. |
Raman spectroscopy of isotopically pure and diluted high- and low-density amorphous ices.
| S. Ishihara, T. Takayama, M. Sakaguchi, T. Otosu, T. Yagasaki, Y. Suzuki, S. Yamaguchi |
J Raman Spectrosc, 53, 1773-1784 (2022). |
| 48. |
Direct observation of reversible liquid-liquid transition in a trehalose aqueous solution.
| Y. Suzuki |
Proc. Natl. Acad. Sci. U.S.A., 119, e2113411119 (2022) |
| 47. |
Polyamorphism of polyol aqueous solutions. (in Japanese)
| Y. Suzuki |
Netsu Sokutei, 49(1), 2-8 (2022) |
| 46. |
Liquid-Phase Transition in Water.
| O. Mishima |
NIMS Monographs, Springer (2021) |
| 45. |
Slow Crystal Growth of Cubic Ice with Stacking Faults in a Glassy Dilute Glycerol Aqueous Solution.
| Y. Suzuki & S. Takeya |
J. Phys. Chem. Lett., 11, 9432-9438 (2020) |
| 44. |
Non-segregated crystalline state of dilute glycerol aqueous solution. (1.2MB)
(Copyright(2016) American Institute of Physics) |
Y. Suzuki |
J. Chem. Phys., 152, 144501 (2020) |
| 43. |
Effect of OH groups on the polyamorphic transition of polyol aqueous solutions. (0.9MB)
(Copyright(2016) American Institute of Physics) |
Y. Suzuki |
J. Chem. Phys., 150, 224508 (2019) |
| 42. |
Experimental estimation of the location of liquid-liquid critical point for polyol aqueous solutions. (1.9MB)
(Copyright(2016) American Institute of Physics) |
Y. Suzuki |
J. Chem. Phys., 149, 204501 (2018) |
| 41. |
Effect of solute nature on the polyamorphic transition in glassy polyol aqueous solutions. (2.8MB)
(Copyright(2016) American Institute of Physics) |
Y. Suzuki |
J. Chem. Phys., 147, 064511 (2017) |
| 40. |
Effect of water polyamorphism on the molecular vibrations of glycerol in its glassy aqueous solutions. (1.0MB)
(Copyright(2016) American Institute of Physics) |
Y. Suzuki, O. Mishima |
J. Chem. Phys., 145, 024501 (2016) |
| 39. |
Experimentally proven liquid-liquid critical point of dilute glycerol-water
solution at 150 K. (1.1MB)
(Copyright(2014) American Institute of Physics) |
Y. Suzuki, O. Mishima |
J. Chem. Phys., 141, 094505 (2014) |
| 38. |
Polyamorphism in water.
A slightly revised version of Proc. Jpn. Acad., Ser. B 86, 165 (2010) |
O. Mishima |
Adv. Chem. Phys., 152, 355 (2013) |
| 37. |
Sudden switchover between the polyamorphic phase separation and the glass-to-liquid transition in glassy LiCl aqueous solutions. (1.1MB)
(Copyright(2013) American Institute of Physics) |
Y. Suzuki, O. Mishima |
J. Chem. Phys., 138, 084507 (2013) |
| 36. |
Melting of the precipitated ice IV in LiCl aqueous solution and polyamorphism of water(2.0MB)
[COVER of J.Phys.Chem.B] |
O. Mishima |
J. Phys. Chem. B 115, 14064-14067 (2011) |
| 35. |
Water polyamorphism and foreseen perspective (in Japanese)(1.6MB) |
Y. Suzuki |
OYO BUTURI 80(10), 890-893 (2011) |
| 34. |
Polarized Raman spectroscopic study on the solvent state of glassy LiCl aqueous solution and the state of relaxed high-density amorphous ices. (0.5MB)
(Copyright(2011) American Institute of Physics) |
Y. Suzuki, Y. Tominaga |
J. Chem. Phys., 134, 244511 (2011) |
| 33. |
Polarized Raman spectroscopic study of relaxed high density amorphous ices under pressure. (0.5MB)
(Copyright(2010) American Institute of Physics) |
Y. Suzuki, Y. Tominaga |
J. Chem. Phys., 133, 164508 (2010) |
| 32. |
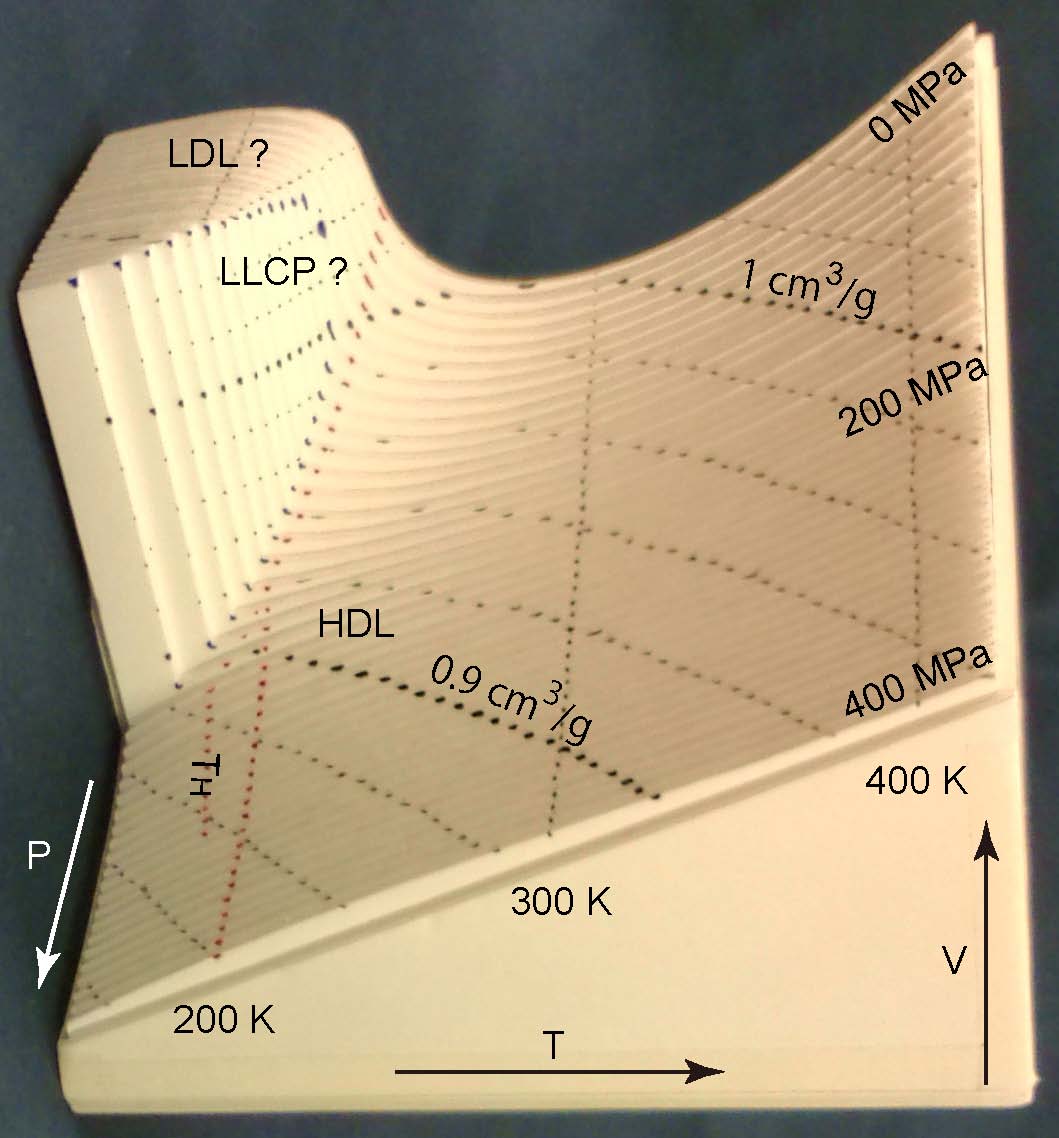
Volume of supercooled water under pressure and the liquid-liquid critical point. (0.7MB) Typo is corrected.[Supplimental material (0.9MB) for the rotation of the 3D figures and the numerical values (Fig. 1). ] |
O. Mishima |
J. Chem. Phys., 133, 144503 (2010) |
| 31. |
Polyamorphism in water. (0.7MB)
A slightly revised version : Adv. Chem. Phys. 152, 355 (2013) |
O. Mishima |
Proc. Jpn. Acad., Ser. B 86, 165-175 (2010) |
| 30. |
Differences between pressure-induced densification of LiCl-H2O glass and polyamorphic transition of H2O. (1.0MB) |
Y. Suzuki, O. Mishima |
J. Phys.: Condens. Matter 21, 155105 (2009) |
| 29. |
Explanation of "the Mysteries of Water" by a Liquid-Liquid Critical Point. (in Japanese) (1.5MB) |
O. Mishima |
Review of High Pressure Science and Technology 17, 352-356 (2007) |
| 28. |
Phase separation in dilute LiCl-H2O solution related to the polyamorphism of liquid water. (1.1MB)
(Copyright(2007) American Institute of Physics) |
O. Mishima |
J. Chem. Phys., 126, 244507 (2007) |
| 27. |
Water polyamorphism - Experimental verification of the validity. (in Japanese) (2.2MB) |
Y. Suzuki |
Butsuri: The Physical Society of Japan, 61, 318-324 (2006) |
| 26. |
Application of a water polyamorphism to an aqueous solution system. (in Japanese) (0.7MB) |
Y. Suzuki |
Low temperature science, 64, 103-113 (2006) |
| 25. |
Application of polyamorphism in water to spontaneous crystallization of emulsified LiCl-H2O solution. (0.3MB)
(Copyright(2005) American Institute of Physics) |
O. Mishima |
J. Chem. Phys., 123, 154506 (2005) |
| 24. |
Evidence of pressure-induced amorphization of tetrahydrofuran clathrate hydrate. |
Y. Suzuki |
Phys. Rev. B, 70, 172108 (2004) |
| 23. |
The glass-to-liquid transition of the emulsified high-density amorphous ice made by pressure-induced amorphization. (0.7MB)
(Copyright(2004) American Institute of Physics) |
O. Mishima |
J. Chem. Phys., 121, 3161 (2004) |
| 22. |
Raman study of the annealing effect of low-density glassy waters. |
Y. Suzuki, O. Mishima |
J. Phys. Soc. Jpn., 72, 3128 (2003) |
| 21. |
Pressure-induced amorphization of ice and polyamorphism in water --Relationship between liquid water and amorphous ices. (in Japanese) (0.2MB) |
O. Mishima |
Review of High Pressure Science and Technology 13, 165-172 (2003) |
| 20. |
Propagation of the polyamorphic transition of ice and the liquid-liquid critical point. (0.5MB) |
O. Mishima & Y. Suzuki |
Nature 419, 599-603 (2002) |
| 19. |
Raman spectroscopic study of glassy water in dilute lithium chloride aqueous solution vitrified under pressure. |
Y. Suzuki, O. Mishima |
J. Chem. Phys., 117, 1673 (2002) |
| 18. |
Vitrification of emulsified liquid water under pressure. |
O. Mishima, Y. Suzuki |
J. Chem. Phys., 115, 4199 (2001) |
| 17. |
Raman spectroscopic study of hyperquenched glassy water in the presence of different non-ionic solute. |
Y. Suzuki |
Chem. Phys. Lett., 335, 357 (2001) |
| 16. |
Low-frequency Raman spectra of amorphous ices. |
Y. Suzuki, Y. Takasaki, Y. Tominaga, O. Mishima |
Chem. Phys. Lett., 319, 81 (2000) |
| 15. |
Liquid-Liquid Critical Point in Heavy Water. |
O. Mishima |
Phys. Rev. Lett., 85, 334 (2000) |
| 14. |
Two distinct Raman profiles of glassy dilute LiCl solution. |
Y. Suzuki, O. Mishima |
Phys. Rev. Lett., 85, 1322 (2000) |
| 13. |
The relationship between liquid,supercooled and glassy water. (0.5MB) |
O. Mishima & H.E Stanley |
Nature 396, 329-335 (1998) |
| 12. |
Decompression-induced melting of ice IV and the liquid-liquid transition in water. (0.4MB) |
O. Mishima & H.E Stanley |
Nature 392, 164-168 (1998) |
| 11. |
Raman spectra of low- and high-density amorphous ices. |
H. Kanno, K. Tomikawa, O. Mishima |
Chem. Phys. Lett., 293, 412 (1998) |
| 10. |
Local structural heterogeneities in liquid water under pressure. |
M. Canpolat, F. W. Starr, A. Scala, M. R. Sadr-Lahijany, O. Mishima, S. Havlin, H. E. Stanley |
Chem. Phys. Lett., 294, 9 (1998) |
| 09. |
Metastable melting lines of ice phases at low temperatures. |
O. Mishima, H. E. Stanley |
Rev. High Pressure Sci. Technol., 7, 1103 (1998) |
| 08. |
Relationship between melting and amorphization of ice.(3.0MB) |
O. Mishima |
Nature 384, 546-549 (1996) |
| 07. |
Reversible first-order transition between two H2O amorphs at 〜0.2 GPa and 〜135 K. |
O. Mishima |
J. Chem. Phys., 100, 5910 (1994) |
| 06. |
Visual observation of the amorphous-amorphous transition in H2O under pressure. |
O. Mishima, K. Takemura, K. Aoki |
Science, 254, 406 (1991) |
| 05. |
High-density amorphous ice. IV. Raman spectrum of the uncouple O-H and O-D oscillators. |
D. D. Klug, O. Mishima, E. Whalley |
J. Chem. Phys., 86, 5323 (1987) |
| 04. |
High-density amorphous ice. III. Thermal properties. |
Y. P. Handa, O. Mishima, E. Whalley |
J. Chem. Phys., 84, 2766 (1986) |
| 03. |
Raman Spectrum of high-density amorphous ice. |
D. D. Klug, O. Mishima, E. Whalley |
Physica B+C, 139 & 140, 475 (1986) |
| 02. |
An apparently first-order transition between two amorphous phases of ice induced by pressure.(2.6MB) |
O. Mishima, L.D. Calvert
& E. Whalley |
Nature 314, 76-78 (1985) |
| 01. |
'Melting' ice I at 77 K and 10 kbar: a new method of making amorphous solids.(2.5MB) |
O. Mishima, L.D. Calvert
& E. Whalley |
Nature 310, 393-395 (1984) |