Researchers Successfully Develop New Chiral Sensing Technique that Enables Easy Determination of Optical Purity
Possible Applications include Enhanced Safety during Drug Synthesisvered
A research team led by MANA Research Associate Jan Labuta and MANA Scientist Jonathan Hill of the Supermolecules Group (Director: Katsuhiko Ariga), in cooperation with ICYS-MANA Researcher Shinsuke Ishihara of the International Center for Young Scientists (Director: Kenjiro Miyano) and researchers from the Charles University in Prague (Czech Republic), has successfully developed a new technique that enables the simple detection of chirality and optical purity. In addition to being simple, the team has demonstrated this technique to be extremely useful and highly versatile.
Like human hands, some molecules possess non-superimposable mirror images of themselves, and this property is called chirality. Despite having the same chemical formula, the properties and bioactivity of organic chiral molecules are completely different. For example, there is a molecule called bupivacaine whose left-handed configuration is effective as an analgesic, but whose right-handed configuration exhibits cardiac toxicity. Therefore, chiral sensing techniques are important tools for determining the chirality or optical purity of molecules.
Optical purity is an important parameter in a chiral molecule because it denotes the relative ratio of right and left-handed configurations. In pharmacology, in addition to quality control, it is important to determine the optical purity at each state of drug synthesis and optimize the manufacturing lines, so drug-makers are seeking simpler and cheaper detection methods.
In this study, the researchers used nuclear magnetic resonance (NMR) and an originally-developed symmetric porphyrin reagent to successfully develop a new technique for detecting optical purity. The most significant feature of this study was its use of a symmetric porphyrin reagent that possesses an achiral structure. Even if this molecule couples with the measured object (i.e., a drug molecule) that is chiral, it does not form a diastereomer, and this is what clearly distinguishes this research from previous existing studies. The mechanism underlying this technique was elucidated using equilibrium binding constants, quantum mechanical calculations and molecular dynamics simulations, based on the fact that the symmetry of the porphyrin reagent would be disrupted when combined with a chiral molecule. The symmetric porphyrin reagent developed for this study is highly versatile and can be used to detect the optical purity of many different chiral molecules, including carboxylic acids, esters, protected amino acids, ketones and alcohols.
Since this technique allows for simple and quick detection of optical purity, it holds promise for use in the pharmaceutical industry. Since the measurement method and reagent used in this technique are achiral, they could be suitable for real-time analyses, including asymmetric synthesis and chiral amplification. To date, conventional methods have been suspected of having an adverse affect on asymmetric synthesis and chiral amplification.
To distinguish the newly developed reagent from conventional reagents obtained from chiral molecules (i.e., NMR chiral shift reagents), the team termed this new chiral sensing molecule a prochiral NMR chiral shift agent (pro-CSA ).
The results of this study were published on the online version of the English scientific journal Nature Communications at 18:00 on July 17, 2013 (10:00 local time, July 17).
The results of this study were published on the online version of the English scientific journal Nature Communications at 18:00 on July 17, 2013 (10:00 local time, July 17).
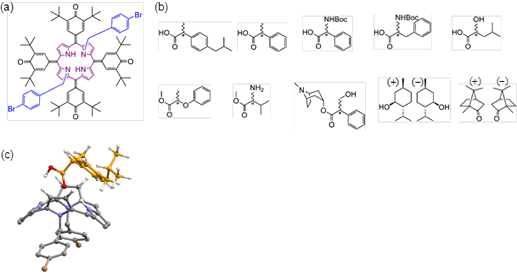
Fig. 1(a): Prochiral NMR chiral shift agent (pro-CSA), the symmetric porphyrin reagent developed for this study. (b): Various chiral molecules whose optical purity can be found using pro-CSA. (c) Diagram of a porphyrin derivative and chiral molecule in a 1:1 complex.
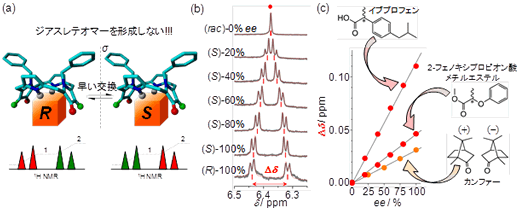
Fig. 2: Operating principles of pro-CSA. (a) Diagram of bonds with chiral guest molecules and NMR spectra. (b) Relationship between the optical purity of ibuprofen and NMR spectra. (c) Linear relationship between the optical purity of chiral molecules (e.e. %) and magnitude of spectral splitting (Δδ).
Further information
Affiliations
Jan Labuta1,Shinsuke Ishihara1,
Tomáš Šikorský2,
Zdeněk Futera3,6,Atsuomi Shundo1,7,
Lenka Hanyková4,Jaroslav V. Burda3,
Katsuhiko Ariga1,5,Jonathan P. Hill1,5
- International Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS)
- CEITEC—Central European Institute of Technology, Masaryk University
- Charles University in Prague, Faculty of Mathematics and Physics, Department of Chemical Physics and Optics
- Charles University in Prague, Faculty of Mathematics and Physics, Department of Macromolecular Physics
- Japan Science and Technology Agency (JST), Core Research for Evolutional Science and Technology (CREST)
- Keio University
- Department of Applied Chemistry, Faculty of Engineering, Kyushu University
Contact information
For more detail about research
Jonathan P. Hill, MANA Scientists, MANA, NIMS
TEL:+81-29-860-4399
E-mail: <Jonathan.Hill nims.go.jp>
nims.go.jp>
TEL:+81-29-860-4399
E-mail: <Jonathan.Hill
 nims.go.jp>
nims.go.jp>Jan Labuta, MANA Research Associates, MANA, NIMS
TEL:+81-29-851-3354
E-mail: <Labuta.Jan nims.go.jp>
nims.go.jp>
TEL:+81-29-851-3354
E-mail: <Labuta.Jan
 nims.go.jp>
nims.go.jp>Shinsuke Ishihara, ICYS-MANA Researcher, MANA, NIMS
TEL:+81-29-851-3354
E-mail: <Ishihara.Shinsuke nims.go.jp>
nims.go.jp>
TEL:+81-29-851-3354
E-mail: <Ishihara.Shinsuke
 nims.go.jp>
nims.go.jp>For general inquiry
Public Relations Office, NIMS
Email: pr nims.go.jp
nims.go.jp
TEL:+81-29-859-2026 FAX:+81-29-859-2017
Email: pr
 nims.go.jp
nims.go.jpTEL:+81-29-859-2026 FAX:+81-29-859-2017




