Interfacial Water Structures and Hydrogen-bond Networks of Major Photocatalytic Systems Are Revealed by First-principles Simulations Using TiO2 Anatase Slabs Dipped in "Bulk" Water
First-principles molecular dynamics using supercells with "bulk" water between the TiO2 anatase (101) and (001) surfaces were carried out for the first time. We showed explicit atomistic structures of strong and weak hydrogen bonds on the TiO2/water interfaces, which had been suggested experimentally so far. The results also give insights into the H2O or OH coverage on the interfaces and their hydrophobicity-hydrophilicity, which is important to understand the photocatalytic mechanisms microscopically.
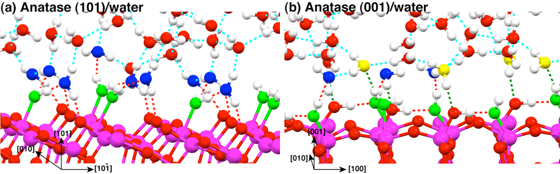
Figure 1: Snapshots of the equilibrium trajectories of the TiO2/bulk H2O interfaces: (a) anatase (101) surface and (b) anatase (001). Red and green dashed lines show strong and weak hydrogen bonds, respectively.
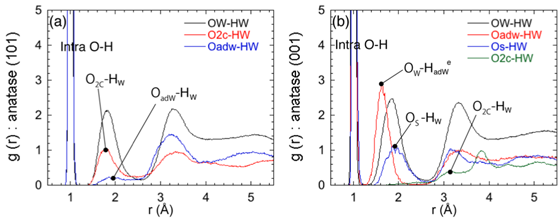
Figure 2: Radial distribution functions from different types of Oxygen to Hydrogen in the present interface systems, showing the presence of strong and weak hydrogen bonds.
Further information
Affiliations
Masato Sumita1, Chunping Hu1,3, Yoshitaka Tateyama1,2
- International Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS)
- Japan Science and Technology Agency
- Present address: Department of Physics, Tokyo University of Science




