 Press Release 2017
Press Release 2017
Activation of Catalytically Inert Material by Formation of Junction with Corrugated 2D Carbon Nitride
- Nanostructurization of Interfaces is the Key. This Strategy May Lead to Understanding of Complex Electrode Processes and Development of Highly Efficient Electrocatalysts -
A NIMS-Hokkaido University joint research team succeeded in activating gold, which is an inert electrocatalyst under certain conditions, by covering it with special framework, thereby nanostructuring its surface. This study was conducted in the context of fuel cell electrode reactions, in which oxygen is converted into water.
(“Two-Dimensional Corrugated Porous Carbon-, Nitrogen-Framework/Metal Heterojunction for Efficient Multielectron Transfer Processes with Controlled Kinetics”, Ken Sakaushi, Andrey Lyalin, Satoshi Tominaka, Tetsuya Taketsugu, and Kohei Uosaki, ACS Nano, 2017, 11 (2), pp 1770–1779, DOI: 10.1021/acsnano.6b07711)
Reactions that produce water from oxygen, or produce hydrogen from water, have been used in energy conversion/storage devices, such as fuel-cell. For example, at the cathode of fuel-cell, oxygen reacts with protons and electrons to produce water (so-called oxygen reduction reaction; ORR). In this reaction, platinum and some oxides are known to serve as effective electrocatalysts. However, due to complex reaction pathways, microscopic mechanism of this reaction is not well understood, even though the reactions seems to be simple, i.e. involving only oxygen, hydrogen and electrons. In this study, we focused on an activation of material by formation of heterojunction with a catalytically inert substance with a special framework in order to clarify the origin of activity and unveil the electrode process. We analyzed electrode processes of this heterojucntioned electrocatalyst to investigate the relationship between the catalyst's chemical/geometrical structures and its catalytic activities.
We formed a heterojunction catalyst by combining graphitic carbon nitride (GCN) with gold. GCN possesses precisely controlled geometric and chemical structures consisting of nitrogen and carbon atoms. After detailed investigation, we found that GCN has a corrugated layered structure, rather than a simple flat layered structure. We isolated a few layers of GCN, attached them to the gold surface, and used this material as an electrocatalyst. It was found that ORR becomes highly efficient if the heterojunction catalyst was used. The computational calculation indicated that the high activity of the heterojunction electrocatalyst may be attributed to the change of gold surface structure at the nanoscale, resulting from the addition of corrugated GCN to the surface, which in turn to create interfacial site to stabilize reaction intermediates-a key part of water synthesis.
These results may facilitate the development of techniques enabling the design of highly efficient and highly selective electrocatalysts for fuel cells. In the future, we plan to study the relationship between a change in the interfacial nanostructure of heterojunction catalysts and their catalytic activities in order to understand reaction processes of many electrodes.
This research was conducted with support of the JSPS Grant-in-Aid for Research Activity Start-up program 15H06850. This research was also supported by the MEXT-commissioned Program for the Development of Environmental Technology using Nanotechnology. In addition, a part of this study was carried out in association with a MEXT FLAGSHIP 2020 project for priority issue 5, titled "Development of new fundamental technologies for high efficiency energy creation, conversion/storage and use."
This study was published in the online version of ACS Nano (known as "ASAP article") on January 30, 2017.
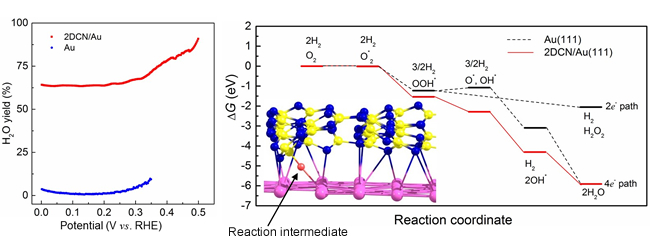
Figure 1 in the press release. (Left) Water formation rate. The heterojunction electrocatalyst (red line) synthesize water much more efficiently than just Au (blue line). (Right) Reaction pathway for gold surface (black line) and the heterojunction electrocatalyst (red line).
(Regarding this research)
Ken Sakaushi
Researcher, Interfacial Energy Conversion Group,
Center for Green Research on Energy and Environmental Materials (C4GR),
National Institute for Materials Science
Tel:+81-29-860-4945
E-Mail:Sakaushi.ken=nims.go.jp
(Please change "=" to "@")
Tetsuya Taketsugu
Professor, Department of Chemistry,
Faculty of Science,
Hokkaido University
Tel:+81-11-706-3535
E-Mail:take=sci.hokudai.ac.jp
(Please change "=" to "@")
Quantum Chemistry Laboratory, Department of Chemistry, Faculty of Science, Hokkaido University
(For general inquiries)
Public Relations Office
National Institute for Materials Science
1-2-1 Sengen, Tsukuba, Ibaraki, 305-0047, JAPAN
Tel:+81-29-859-2026
Fax:+81-29-859-2017
E-Mail:pressrelease=ml.nims.go.jp
(Please change "=" to "@")
General Affairs and Planning Department
Hokkaido University
Tel:+81-11-706-2610
Fax:+81-11-706-2092
E-Mail:kouhou=jimu.hokudai.ac.jp
(Please change "=" to "@")

