 Press Release 2013
Press Release 2013
Carbon dioxide "breathing phenomenon" found in clay minerals
Expectations for application to efficient carbon dioxide separation membranes, etc.
Dr. Shinsuke Ishihara, ICYS-MANA Researcher at the ICYS in MANA, NIMS, Dr. Nobuo Iyi, NIMS Special Researcher at MANA, NIMS, and collaborators discovered a new phenomenon whereby carbonate ions in a clay mineral called "hydrotalcite" are exchanged with carbon dioxide in the air, quickly over several days, as if the clay mineral is breathing carbon dioxide.
Dr. Shinsuke Ishihara, ICYS-MANA Researcher at the International Center for Young Scientists (ICYS) (Managing Director: Kenjiro Miyano) in the International Center for Materials Nanoarchitectonics (MANA) (Director-General: Masakazu Aono) of the National Institute for Materials Science (NIMS) (President: Sukekatsu Ushioda), Dr. Nobuo Iyi, NIMS Special Researcher at the International Center for Materials Nanoarchitectonics (MANA) (Director-General: Masakazu Aono), NIMS, and collaborators discovered a new phenomenon whereby carbonate ions (CO32−) in a clay mineral called "hydrotalcite" are repeatedly exchanged with carbon dioxide (CO2) in the air, quickly over several days, as if the clay mineral is breathing carbon dioxide.
Due to concerns over global warming and other issues, much attention has been focused on better understanding of the global carbon cycle. Carbon (C) is not only found in carbon dioxide in the air or sea, but it is also cycled at a global scale through the life activities of plants and animals. Rocks called "carbonate rocks" store the largest amount of carbon on the Earth (approx. 60 quadrillion tons), far exceeding the amounts contained in the air (720 billion tons) and in the sea (38 trillion tons). Meanwhile, it had been believed that it takes millions of years for carbonate rocks to weather and return to the global carbon cycle.
This time, we used hydrotalcite containing 13C isotope-labeled carbonate ions (13CO32−), and observed for the first time that carbonate ions in the hydrotalcite are exchanged with carbon dioxide in the air in a time span of several days to one week. This means we have discovered a material group that dramatically changes the conventional concept of the carbon cycle. Since 12C constitutes 98.9% of the carbon contained in the carbon dioxide in the air, it can be distinguished from 13C using infrared spectroscopy.
Results of gaseous adsorption tests evidenced the presence of interlayer sites in hydrotalcite that selectively absorb up to about 4cc/g of carbon dioxide. Interestingly, nitrogen gas with a smaller molecule diameter than carbon dioxide cannot enter this interlayer site. By making full use of theoretical chemistry (first principles calculation and density-functional-theory calculation) and nuclear magnetic resonance spectroscopy, we have obtained detailed knowledge on the mechanism of carbon dioxide absorption, exchange and desorption with regard to hydrotalcite.
Because clay minerals similar to hydrotalcite also exist in nature, this research result is considered to contribute to a more accurate understanding of global warming related to the carbon cycle and of radiocarbon dating methods. We also expect that, by increasing the amount of absorption and the exchange speed of carbon dioxide through structural optimization of hydrotalcite, we can develop next-generation materials such as efficient carbon dioxide separation membranes and carbon dioxide reduction catalyst supports.
This research result has been published in Journal of the American Chemical Society, a journal issued by the American Chemical Society.
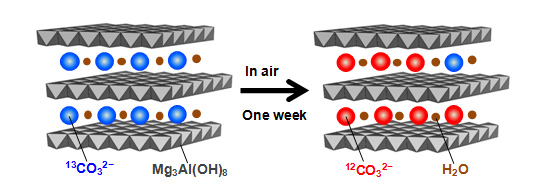
Exchange between carbonate ions in hydrotalcite and carbon dioxide in the air
For more detail about research
Shinsuke Ishihara ICYS-MANA Researcher, MANA, NIMS
Tel:+81-29-851-3354
E-Mail:Ishihara.Shinsuke=nims.go.jp
(Please change "=" to "@")

