 Press Release 2012
Press Release 2012
Atom Resolved Imaging of an Electrochemical Reaction
-Revealing the physical and chemical phenomena which are indispensable for developing highly efficient ionic devices-
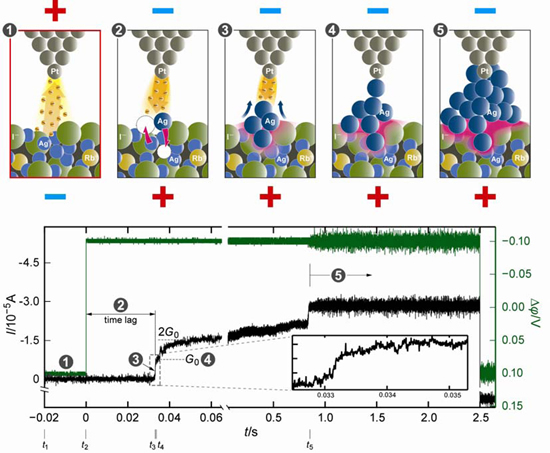
Fig. 1 Solid electrochemical reaction process. (a) Schematic illustration of the process. The process is categorized into five regions. 1) STM observation using a bias which does not cause the solid electrochemical reaction. 2) Electron injection into the ionic material by changing the bias polarity. 3) Precipitation of atoms starts after a certain time. 4) Precipitated atoms form a bridge connected to the counter electrode. 5) The bridge thickens due to the further precipitation of atoms. (b) Experimental result. Change in the applied bias (green) and the measured current (black). The number of precipitated atoms can be estimated from the change in the current.
Further information
Affiliation
- 1.Research Center Juelich
- 2.IWE2 & JARA-FIT, RWTH Aachen
- 3.International Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS)
- 4.Institute of Theoretical Chemistry, University of Bonn

