代表的な研究成果:
 [Informatics, Battery]
[Informatics, Battery]
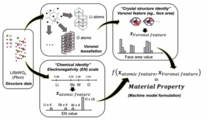
To achieve high predictive accuracy of target properties on trained machine models for use in large-scale material screening, a general material representation that can effectively encode characteristics of crystalline solids or uniquely differentiate among them in terms of chemical composition, crystal structure, etc., is highly requested. Encoding crystalline solids based on structural descriptors of directly binned Voronoi-tessellation real feature values and atomic/chemical descriptors based on the electronegativity of elements in the crystal. A new general representation scheme for crystalline solids that lifts restrictions on atom ordering, cell periodicity, and system cell size was realized and validated.
Randy Jalem*, Yoshitaka Tateyama et al., Sci. Tech. Adv. Mater.19, 231-242 (2018). “A General Representation Scheme for Crystalline Solids based on Voronoi-Tessellation Real Feature Values and Atomic Property Data”
 [Kinetics, Catalyst]
[Kinetics, Catalyst]
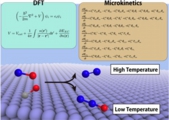
We have addressed first-principles “surface” micro kinetics study, together with reactor models on a catalytic reaction, and evaluated the conversion rate and selectivity with a certain precision. This sort of computational investigation is still rare and needs the assessment, to which this paper surely contributes.
Atsuhi Ishikawa* , Yoshitaka Tateyama*, J. Phys. Chem. C 122, 17378-17388 (2018). "First-Principles Microkinetic Analysis of NO + CO Reactions on Rh(111) Surface toward Understanding NOx Reduction Pathways"
 [Na-excess cathode, O redox, Battery]
[Na-excess cathode, O redox, Battery]
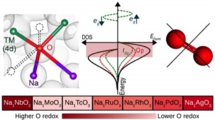
We have carried out first first-principles calculation study on the Na-excess cathode materials (NaxTMO3: x=0.5-2, TM=4d) and the elucidated the mechanism. Then, further examining the other 4d transition metal oxide, we proposed NaxNbO3 can be a candidate for better characteristics.
M. H. N. Assadi, Yoshitaka Tateyama* et al., J. Mater. Chem. A 6, 3747-3753 (2018). “Oxygen redox in hexagonal layered NaxTMO3 (TM =4d elements) for high capacity Na ion batteries”
 [Superconcentrated electrolyte, Battery]
[Superconcentrated electrolyte, Battery]
Together with Prof. Yamada group in The Univ. of Tokyo, we have investigated microscopic characters and mechanisms of super concentrated electrolytes such as LiTFSA/AN, Hydrate melt, fire-extinguisher electrolyte etc., and been providing novel aspects regarding the battery electrolytes.
Wang Jianhui, Keitaro Sodeyama, Yoshitaka Tateyama, Atsuo Yamada et al., Nat. Energy 3, 22-29 (2018). “Fire-extinguishing organic electrolytes for safe batteries”: Yuki Yamada, Keitaro Sodeyama, Yoshitaka Tateyama, Atsuo Yamada et al., Nat. Energy 1, 16129 (2016). “Hydrate-melt electrolytes for high-energy-density aqueous batteries”: Jianhui Wang, Yuki Yamada, Keitaro Sodeyama, Yoshitaka Tateyama, Atsuo Yamada et al., Nat. Commun. 7, 12032 (2016). “Superconcentrated electrolytes for a high-voltage lithium-ion battery”
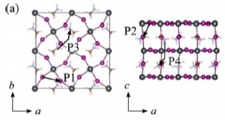 [Ion migration (cation & anion), Degradation, Perovskite solar cell]
[Ion migration (cation & anion), Degradation, Perovskite solar cell]
To understand the origins of the I-V curve hysteresis and degradation, we examined the anion and cation migration (with vacancy scheme) in MAPBI3 and FAPBI3. Our results indicate that the cations can migrate (the barrier around 0.6 eV) as well as the anion (the barrier around 0.2-0.3 eV), which is a probable reason for the hysteresis and degradation of the structure. We also proposed that the inclusion of large FA cations can suppress the cation migration, leading to better stability.
Jun Haruyama, Yoshitaka Tateyama* et al., J. Am. Chem. Soc. 137, 10048-10051 (2015). “First-Principles Study of Ion Diffusion in Perovskite Solar Cell Sensitizers”
 [Interface electron/hole transfer, Perovskite solar cell]
[Interface electron/hole transfer, Perovskite solar cell]
We carried out the first-principles calculation studies on the energetics and electronics states of the surface and the interface with TiO2 of MAPBI3 perovskite materials. These investigations provided novel aspects about the interfacial electron / hole transfer. In addition, the studies demonstrated the shallow donor / acceptor surface & defects states, leading to longer carrier transport and the narrow stable region of MAPBI3 w.r.t. the chemical potential of I and Pb.
Jun Haruyama*, Yoshitaka Tateyama* et al., J. Phys. Chem. Lett. 8, 5840-5847 (2017). “First-Principles Study of Electron Injection and Defects at the TiO2/ CH3NH3PbI3 Interface of Perovskite Solar Cells”: Jun Haruayama, Yoshitaka Tateyama* et al., Acc. Chem. Res. 49, 554-561 (2016). “Surface Properties of CH3NH3PbI3 for Perovskite Solar Cells” : Jun Haruyama, Yoshitaka Tateyama* et al., J. Phys. Chem. Lett. 5, 2903-2909 (2014). “Termination Dependence of Tetragonal CH3NH3PbI3 Surfaces for Perovskite Solar cells”
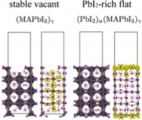
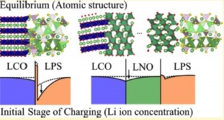 [Solid electrolyte - electrode interface, Ion depletion growth, Solid state battery]
[Solid electrolyte - electrode interface, Ion depletion growth, Solid state battery]
We investigated the microscopic origins of the interfacial resistance at the sulphide electrolyte and the oxide cathode interfaces by first-principles calculations. Performing a systematic search for the structures of solid-solid interfaces, we obtained the (meta) stable structures and the electronics states. The chemical potential of Li then indicated that, upon charging, the Li depletion will grow around the electrolyte sites on the interface, leading to suppression of bunching Li-ion transport. We concluded that the space-charge layer effect proposed before is actually the growth of Li depletion under operation on the atomic scale.
Jun Haruyama, Yoshitaka Tateyama* et al., ACS Appl. Mater. Interfaces 9, 286-292 (2017). “Cation Mixing Properties toward Co Diffusion at the LiCoO2 Cathode/ Sulfide Electrolyte Interface in a Solid-State Battery”: “Space-Charge Layer Effect at Interface between Oxide cathode and Sulfide Electrolyte in All-Solid-State Lithium-Ion Battery”, Jun Haruyama, Yoshitaka Tateyama* et al., Chem. Mater. 26, 4248-4255 (2014).
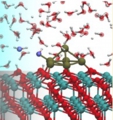 [CeO2/Pt/H2O interface, Interfacial proton transfer, Catalyst]
[CeO2/Pt/H2O interface, Interfacial proton transfer, Catalyst]
For several catalytic reactions such as water gas shift, we have investigated the water adsorption, water dissociation, and proton / OH- transfer as well as the interfacial charge transfer at the CeO2/Pt cluster/H2O interfaces by first-principles molecular dynamics calculations. We then demonstrated fast H+/OH- transfer near the interface as well as oxidation of the Pt cluster.
Matteo Farnesi Camellone, Lucie Szabova, Yoshitaka Tateyama, Stefano Fabris et al., J. Am Chem. Soc. 138, 11560-11567 (2016). “Catalytic Proton Dynamics at the Water/Solid Interface of Ceria Supported Pt Clusters”: Lucie Szabova, Yoshitaka Tateyama, Stefano Fabris et al., J. Phys. Chem. C 119, 2537-2544 (2015). “Water Adsorption and Dissociation at Metal-Supported Ceria Thin Films: Thickness and Interface-Proximity Effects Studied with DFT+U Calculations”,
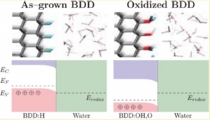 [Boron-dope diamond electrode, Space-charge layer, Catalyst]
[Boron-dope diamond electrode, Space-charge layer, Catalyst]
We examined the boron-doped diamond (BDD) electrode - water interfaces with different terminations. We then demonstrated that H-terminated BDD has a higher band position, leading to negative electron affinity, while the oxidised BDD has a lower position, leading to higher oxidation ability. We then proposed how to understand CV behaviours of redox reactions with BDD electrode based on the electron / hole tunnelling in the subsurface space-charge layer.
Takeshi Kashiwada, Yusuke Ootani, Yoshitaka Tateyama, Yasuaki Einaga et al., ACS Appl. Mater. Interfaces (2016). “A Study on Electrolytic Corrosion of Boron-Doped Diamond Electrodes when Decomposing Organic Compounds” : Zdenek Futera, Yoshitaka Tateyama* et al., J. Phys. Chem. C, 118, 22040-22052 (2014). "First Principles Calculation Study on Surfaces and Water Interfaces of Boron-Doped Diamond”
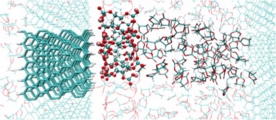 [Reductive decomposition of electrolyte, SEI film formation & characteristics, Battery]
[Reductive decomposition of electrolyte, SEI film formation & characteristics, Battery]
We addressed understanding the microscopic mechanism of SEI (solid electrolyte interphase) film at liquid electrolyte - anode interfaces in battery, by using first-principle molecular dynamics samplings and free energy evaluations. We then found a novel mechanism of additive effect, passivation not sacrificial decomposition for the typical EC solvent/VC additive electrolyte. Then, we also proposed a new mechanism for formation of organic SEI, “near-shore aggregation mechanism”, which can explain all the experimental observation so far. We further demonstrated the glue roles of Li and F in the SEI film. These theoretical proposals stimulated the atomistic investigations of the SEI film.
Yukihiro Okuno, Keisuke Ushirogata, Keitaro Sodeyama, Yoshitaka Tateyama*, Phys. Chem. Chem. Phys. 18, 8643-8653 (2016). “Decomposition of the fluoroethylene carbonate additive and the glue effect of lithium fluoride products for the solid electrolyte interphase: an ab initio study”: Keisuke Ushirogata, Keitaro Sodeyama, Yoshitaka Tateyama*, Yukihiro Okuno* et al., J. Electrochem. Soc. 162, A2670-A2678 (2015). “Near-Shore Aggregation Mechanism of Electrolyte Decomposition Products to Explain Solid Electrolyte Interphase Formation”: Keisuke Ushirogata, Keitaro Sodeyama, Yukihiro Okuno, Yoshitaka Tateyama*, J. Am. Chem. Soc. 135, 11967-11974 (2013). "Additive Effect on Reductive Decomposition and Binding of Carbonate-Based Solvent toward Solid Electrolyte Interphase Formation in Lithium-Ion Battery"
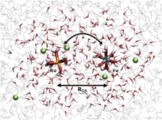 [Theory, Methodology, Redox potential]
[Theory, Methodology, Redox potential]
Based on the combination of the Marcus theory and DFT molecular dynamics sampling, we developed a general DFT-level computational method for donor-acceptor electron transfer reactions, which allows us at which distance the electron transfer occurs. The method is a sort of QM/MM method including two QM regions for donor and acceptor, which was named “double QM/MM method”. In principle, this concept can be applied for any donor-acceptor electron transfer including the interfacial one.
Zdenek Futera, Yoshitaka Tateyama* et al., Phys. Chem. Chem. Phys. 16, 19530-19539 (2014). “A double-QM/MM method for investigating donor-acceptor electron-transfer reactions in solution”: Ryota Jono, Yoshitaka Tateyama, Koichi Yamashita, Phys. Chem. Chem. Phys. 17, 27103-27108 (2015). “A method to calculate redox potentials relative to the normal hydrogen electrode in nonaqueous solution by using density functional theory-based molecular dynamics”: Ryota Jono, Yoshitaka Tateyama, Koichi Yamashita, J. Phys. Chem. Lett. 3, 3581-3584 (2012). “Redox Reaction Mechanisms with Non-triiodide Mediators in Dye-Sensitized Solar Cells by Redox Potential Calculations”
[Superconcentrated electrolyte, Battery]
“Sacrificial anion reduction mechanism for electrochemical stability improvement in highly concentrated Li-salt electrolyte”, Keitaro Sodeyama, Yuki Yamada, Koharu Aikawa, Atsuo Yamada, Yoshitaka Tateyama, J. Phys. Chem. C 118, 14091-14097 (2014).
"Unusual Stability of Acetonitrile-Based Superconcentrated Electrolytes for Fast-Charging Lithium-Ion Batteries", Y. Yamada, K. Furukawa, K. Sodeyama, K. Kikuchi, M. Yaegashi, Y. Tateyama, and A. Yamada, J. Am. Chem. Soc. 136 (2014):
[Water configuration, Swelling]
"Unusually Stable ~100-Fold Reversible and Instant Swelling of Inorganic Layered Materials", F. Geng, R. Ma, A. Nakamura, K. Akatsuka, Y. Ebina, Y. Yamauchi, N. Miyamoto, Y. Tateyama, and T. Sasaki, Nat. Commun. 4, 1632 (2013).
[TiO2/water interface, TiO2/organic electrolyte interface, Catalyst, Solar cell]
“Water contamination effect on liquid acetonitrile / TiO2 anatase (101) interface for durable dye-sensitized solar cell”, Masato Sumita, Keitaro Sodeyama, Liyuan Han, Yoshitaka Tateyama, J. Phys. Chem. C 115, 19849-19855 (2011).
[Theory, Method, DFT-MD, Marcus theory, Thermodynamic integration, Redox potential]
“Free energy calculation of water addition coupled to reduction of aqueous RuO4-”, Yoshitaka Tateyama, Jochen Blumberger, Takahisa Ohno, Michiel Sprik, J. Chem. Phys. 126, 204506 (2007):“Density-functional molecular-dynamics study of the redox reactions of two anionic, aqueous transition-metal complexes”, Yoshitaka Tateyama, Jochen Blumberger, Michiel Sprik, Ivano Tavernelli, J. Chem. Phys. 122, 234505 (2005).
[Excited states dynamics, Method]
“Real-time propagation time-dependent density functional theory study on the ring-opening transformation of the photoexcited crystalline benzene”, Yoshitaka Tateyama, Norihisa Oyama, Takahisa Ohno, Yoshiyuki Miyamoto, J. Chem. Phys. 124, 124507 (2006).
[Hydrogen embrittlement, Hydrogen, Defect, Iron]
“Stability and clusterization of hydrogen-vacancy complexes in alpha-Fe: An ab-initio study”, Yoshitaka Tateyama, Takahisa Ohno, Phys. Rev. B67, 174105 (2003): “Atomic-scale effects of hydrogen in iron toward hydrogen embrittlement: Ab-initio study”, Yoshitaka Tateyama, Takahisa Ohno, ISIJ Int. 43, 573 (2003)


