 Press Release 2023
Press Release 2023
Non-Catalytic Approach to Accelerating Chemical Reactions Using Clay
A research team consisting of the National Institute for Materials Science (NIMS) and the University of Queensland has discovered a new method of accelerating chemical reactions using clay nanosheets without relying on heat energy or rare metal-containing catalysts.
“Extraordinary Acceleration of an Electrophilic Reaction Driven by the Polar Surface of 2D Aluminosilicate Nanosheets"
Nagy Torad, Yuta Tsuji, Azhar Alowasheeir, Masako Momotake, Kazuki Okazawa, Kazunari Yoshizawa, Michio Matsumoto, Masafumi Yamato, Yusuke Yamauchi, and Miharu Eguchi;
Journal: Small [January 9, 2023]
DOI : 10.1002/smll.202205857
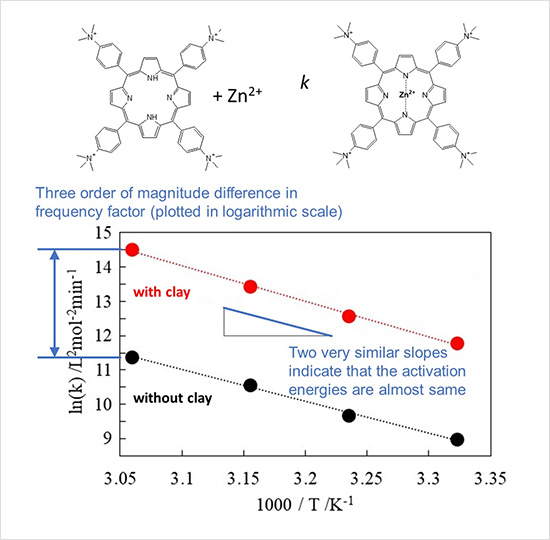
Figure.
(Top) The complexation of a porphyrin molecule with a zinc ion.
(Bottom) Rate constants, k, at various temperatures with (red circles) and without clay (black circles).

Miharu Eguchi

Michio Matsumoto

Yusuke Yamauchi
Abstract
Chemical reactions can be accelerated by raising the temperature, increasing the reactant concentrations or using catalysts—substances capable of increasing the rate of chemical reactions by reducing the amount of activation energy needed to trigger them. However, many chemical reactions are incompatible with these approaches because some reactants are unstable at high temperatures, while others cannot be prepared at high concentrations due to their poor solubility in solvents. In addition, suitable catalysts are not necessarily available for all the reactions of interest. The discovery of other effective methods of accelerating these reactions would be welcomed.
This research team focused on a specific type of porphyrin— a macrocyclic organic molecule with its active site at its center, as in hemoglobin in red blood cells—and a chemical reaction in which a zinc ion binds to this porphyrin. The team has found that porphyrin attached to clay reacts with zinc ion 23-times faster than porphyrin alone. The team also found that the clay nanosheets did not act as a catalyst (i.e., they did not reduce the amount of activation energy needed to drive the zinc–porphyrin reaction). The temperature-dependence was virtually the same in both as indicated by the essentially the slopes of the two lines in the graph, showing that the clay nanosheets have no catalytic activity. Despite the non-catalytic nature of the clay nanosheets, the frequency factor (i.e., the frequency of properly oriented collisions between porphyrins and zinc ions) increased by almost three orders of magnitude when the porphyrins were attached to them, as indicated by the y-intercepts of the two lines in the graph. Various spectroscopic measurements revealed that when one side of a porphyrin attaches to a negatively charged clay nanosheet surface via adsorption, the electron density (i.e., the negative charge density) on the other side of the porphyrin away from the nanosheet surface increases. This electric polarization conceivably increases the frequency of positively charged zinc ions coming into contact with negatively charged porphyrin surfaces via electrostatic attraction, thereby speeding up the chemical reaction.
This nanosheet material capable of accelerating chemical reactions is made of clay, a ubiquitous substance which can be harvested without significantly impacting the environment. The research team will work to improve this technique so that it can be used to speed up more complex chemical reactions of greater practical importance. The team also plans to explore non-clay nanosheet materials that may be similarly effective in expediting chemical reactions.
This project was carried out by an international joint research team consisting of Miharu Eguchi (Senior Researcher, Mesoscale Materials Chemistry Group, International Center for Materials Nanoarchitectonics, NIMS) and a researcher from The University of Queensland. This work was supported in part by JST-ERATO Yamauchi Materials Space-Tectonics Project.
This project was carried out as part of the Yamauchi Materials Space-Tectonics Project funded by the Japan Science and Technology Agency (JST)’s Exploratory Research for Advanced Technology (ERATO) program.
This research was published in the online version of Small.
(Regarding this research)
Miharu Eguchi
Senior Researcher
Mesoscale Materials Chemistry Group, MANA, NIMS
Tel:+81-29-860-4978
E-Mail:eguchi.miharu=nims.go.jp=nims.go.jp
(Please change "=" to "@")
【Inquiries after April 1, 2023】
Miharu Eguchi
Associate Professor
Waseda University
E-Mail:eguchi=waseda.jp
(Please change "=" to "@")
(General information)
Public Relations Office
National Institute for Materials Science
Tel: +81-29-859-2026
Fax: +81-29-859-2017
E-Mail: pressrelease=ml.nims.go.jp
(Please change "=" to "@")
(Inquiries about the JST’s ERATO research funding program)
Yusuke Yamauchi
ERATO Project Director
Professor, University of Queensland
Group Leader, National Institute for Materials Science
E-Mail:y.yamauchi=uq.edu.au
(Please change "=" to "@")

