RESEARCH
6. Tetrapyrrolic Macrocycles and Phenols for Sensing Applications
The combination of calix[4]pyrrole macrocycle with electronically conjugated meso-substituents leads to a highly chromogenic molecule which not only responds to a variety of guests by undergoing a colour change but also can be synthetically modified to include other functionalities including porphyrins or fullerenes. These compounds can be used to detect anions, solvents (including water), acidity or basicity, and even enantiomeric excesses of many chiral compounds.
Sensing of Enantiomeric Excess
Recently we have discovered a novel method of determining enantiomeric excesses (ee) of chiral compounds using a saddle-shaped oxidized porphyrin macrocycle (which we refer to as OxP). A simple proton nuclear magnatice resonance protocol has been developed enabling ee of a great variety of chiral compounds including pharmaceuticals, natural products and synthetic intermediates.
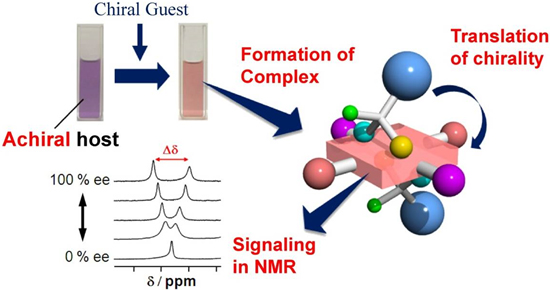
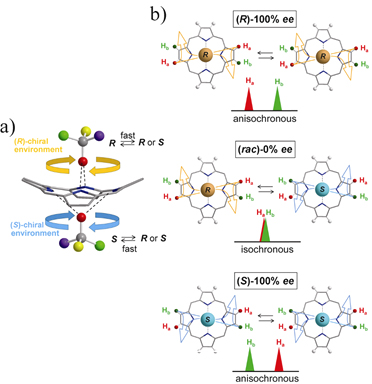
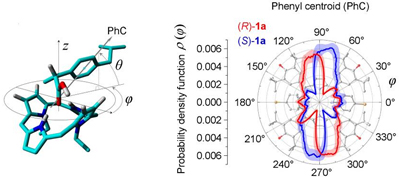
Also recently, we have found that enantiomeric excess can be evaluated using NMR spectroscopy without the formation of diastereomeric species in solution. An extensive mechanistic investigation was also made.
Other Aspects of Sensing using Tetrapyrroles
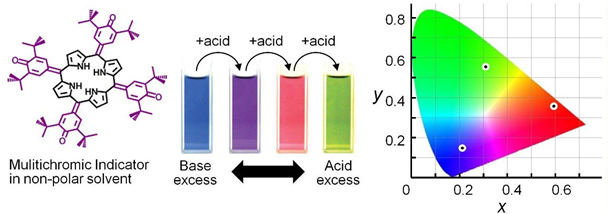
The same saddle-shaped OxP as above can also be used to detect anions, solvents, water and can even be used to estimate acidity in non-polar solvents.
Sensing of Deleterious Metal Cations
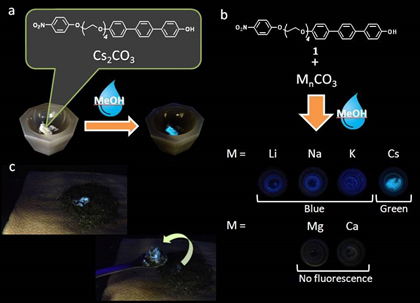
In the wake of the Fukushima disaster it has become imperative that simple methods for detecting deleterious materials following nuclear reactor breaches are developed. In this context, we have developed a fluorogenic sensing system for detection of caesium cations contained in particulates, which are contaminants of ground soils. Caesium is not a common element in the Earth’s crust but its prevalence may increase in the locality of reactor breaches. Mapping of radiocaesium contaminated land will assist local clean-up operations.
Related publications
- J. Labuta, S. Ishihara, T.Šikorský, Z. Futera, A. Shundo, L. Hanyková, J. V. Burda, K. Ariga and J. P. Hill. “NMR Spectroscopic Detection of Chirality and Enantiopurity in Referenced Systems without Formation of Diastereomers” Nature Communications (2013) DOI: 10.1038/ncomms3188. [Link
 ]
] - A. Shundo, S. Ishihara, J. Labuta, Y. Onuma, H. Sakai, M. Abe, K. Ariga and J. P. Hill. “Colorimetric Visualization of Acid-base Equilibria in Non-polar Solvent” Chem. Commun. (2013) 49, 6870–6872. [Link
 ]
] - S. Ishihara, N. Iyi, J. Labuta, K. Deguchi, S. Ohki, M. Tansho, T. Shimizu, Y. Yamauchi, M. Naito, H. Abe, J. P. Hill and K. Ariga. “Naked-eye Discrimination of Methanol and Ethanol using Composite Film of Oxoporphyrinogen and Layered Double Hydroxide” ACS Applied Materials and Interfaces (2013) 5, 5927–5930. [Link
 ]
] - T. Mori, M. Akamatsu, K. Okamoto, M. Sumita, Y. Tateyama, H. Sakai, J. P. Hill, M. Abe and K. Ariga. “Micrometer-level Naked Eye Detection of Caesium Particles in the Solid State” Sci. Tech. Adv. Mater. (2013) 14, 015002. [Link
 ]
] - S. Ishihara, J. Labuta, T. Šikorský, J. V. Burda, N. Okamoto, H. Abe, K. Ariga and J. P. Hill. “Colorimetric Detection of Trace Water in Tetrahydrofuran Using N,N’-Substituted Oxoporphyrinogens” Chem. Commun. (2012) 48, 3933–3935. [Link
 ]
] - J. P. Hill, N. K. Subbaiyan, F. D’Souza, Y. Xie, S. Sahu, N. M. Sanchez-Ballester and K. Ariga. “Antioxidant-substituted Tetrapyrazinoporphyrazine as Fluorescent Sensor for Basic Anions” Chem. Commun. (2012) 48, 3951–3953. [Link
 ]
] - Y. Xie, Y. Ding, C. Wang, J. P. Hill, K. Ariga, W. Zhang and W. Zhu. “Selective, Sensitive and Reversible “Turn-on” Fluorescent Sensors of Cyanide Anions based on 2,2’-Dipyridylaminoanthracene-Cu2+ Ensembles” Chem. Commun. (2012) 48, 11513–11515. [Link
 ]
] - K. Sakakibara, L. A. Joyce, T. Mori, T. Fujisawa, S. H. Shabbir, J. P. Hill, E. V. Anslyn and K. Ariga “Mechanically-Controlled Indicator Displacement Assay (MC-IDA)” Angew.Chem. Int. Ed. (2012) 51, 9643–9646. [Link
 ]
] - J. Labuta, S. Ishihara, A. Shundo, S. Arai, S. Takeoka, K. Ariga and J. P. Hill. “Chirality Sensing by Non-chiral Porphines” Chem. Eur. J. (2011) 17, 3558–3561. [Link
 ]
] - Y. Ding, Y. Xie,X. Li,J. P. Hill,W. Zhang,and W. Zhu. “Selective and Sensitive "Turn-on'' Fluorescent Zn2+ Sensors based on Di- and Tripyrrins with Readily Modulated Emission Wavelengths” Chem. Commun. (2011) 47, 5431–5433. [Link
 ]
] - K. Ariga, G. J. Richards, S. Ishihara, H. Izawa and J. P. Hill. “Intelligent Chiral Sensing based on Supramolecular and Interface Concepts” Sensors (2010) 10, 6796–6820. [Link
 ]
] - A. Shundo, J. Labuta, J. P. Hill, S. Ishihara and K. Ariga. “Nuclear Magnetic Resonance Signaling of Molecular Chiral Information using an Achiral Reagent” J. Am. Chem. Soc. (2009) 131, 9494–9495. [Link
 ]
] - A. Shundo, J. P. Hill and K. Ariga. “Toward Volatile and Nonvolatile Memories: Fluorescence Switching Based on Specific Fluoride-Triggered Interconversion of Simple Porphyrin Derivatives” Chem. Eur. J. (2009) 15, 2486–2490. [Link
 ]
] - A. L. Schumacher, J. P. Hill, K. Ariga and F. D’Souza. “Highly Effective Anion Sensing based on Oxoporphyrinogen” Electrochem, Commun. (2007) 9, 2751–2754. [Link
 ]
] - Y. Xie, J. P. Hill, R. Charvet and K. Ariga. “Porphyrin Colorimetric Indicators in Molecular and Nano-Architectures” J. Nanosci. Nanotechnol. (2007) 7, 2969–2993. [Link
 ]
] - J. P. Hill, A. L. Schumacher, F. D’Souza, J. Labuta, C. Redshaw, M. J. R. Elsegood, M. Aoyagi, T. Nakanishi and K. Ariga. “A Chromogenic Sensor for Anion Reporting based on an N-Substituted Oxo-porphyrinogen”Inorg. Chem. (2006) 45, 8288–8296. [Link
 ]
]