Battery Interface Control Group
Menber
OHNISHI Tsuyoshi
SAMURAI
Group Leader, Battery Interface Control Group, Battery and Cell Materials Field, Research Center for Energy and Environmental Materials (GREEN)

MIYOSHI Shogo
SAMURAI
Senior Researcher, Battery Interface Control Group, Battery and Cell Materials Field, Research Center for Energy and Environmental Materials (GREEN)

Motivation and Outline
Some solid materials show ionic conduction like liquid electrolytes, which are called solid electrolytes. Application of solid electrolytes to lithium-ion batteries is expected to offer longer life and overcome safety issues, which originates from use of combustible organic electrolytes in current lithium-ion batteries. Studies in Battery Interface Control Group are aiming at the development of such solid-state batteries with focusing phenomena among homo- and hetero-interfaces.
Facilities
Our facilities for the investigations of interface phenomena in oxide all-solid-state battery include apparatuses for sample preparation via vapor deposition and sintering techniques, and characterization for structure and electrochemical analyses.

Research Results
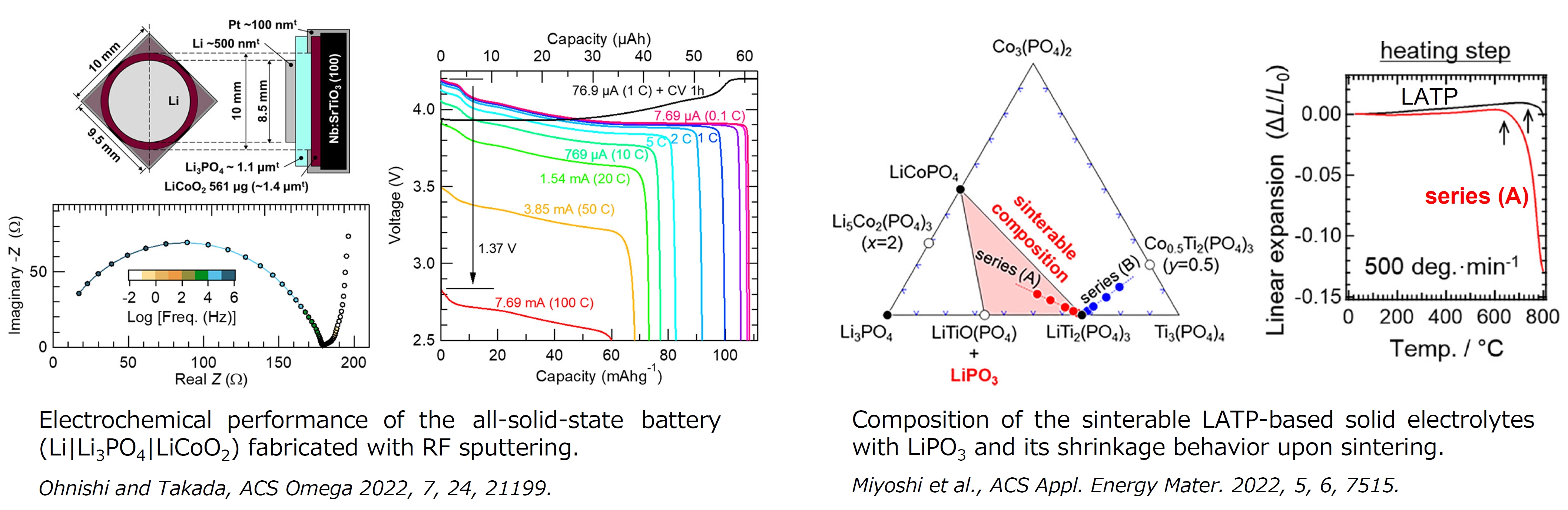
We have working on research and development of all-solid-state Li-ion batteries with oxide electrolytes by employing thin-film technologies, leading to development of fabrication processes for superior batteries, surface treatment techniques to improve interface properties, and so on. The left figure indicates the results that the Li3PO4 films prepared with RF sputtering show good ionic conductivity, and the battery with the Li3PO4 film electrolyte is capable of quite high discharge rate.
The other results are related to sintering process of oxide solid electrolytes, which is the standard technique for achieving interface joining of ceramics. The work involves suggestion of sintering aids and mechanism of sintering promotion on the basis of equilibrium phase relationships. The right figure shows that sintering of Al-doped LiTi2(PO4)3 (LATP), which is a NASICON-type solid electrolyte, is promoted by equilibrium formation of a low-melting-point compound, LiPO3.