'Micrometre-level naked-eye detection' of caesium ions, a major source of contamination in the vicinity of radioactive leaks, is demonstrated in a material developed by researchers in Japan.
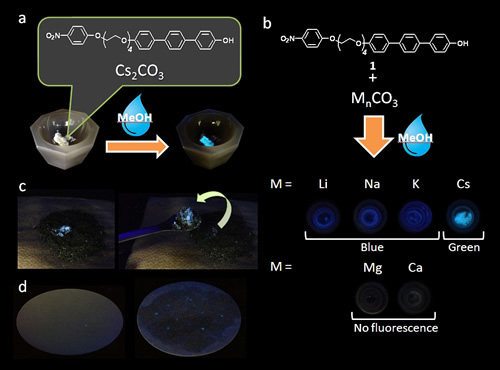
Figure : Photographs of fluorescence changes of a mixture of the phenol compound and various carbonate salts after addition of a drop of methanol. (a) Fluorescence change of a powdered mixture of the compound and Cs2CO3 under UV irradiation (365 nm) after addition of a drop of methanol. (b) Photographs of a mixture of the compound and various carbonate salts of other metals under UV irradiation (365 nm) after addition of a drop of methanol. (c) Photographs of the compound with caesium cations on dirt under room light (left) and under UV irradiation (365 nm, right) after spraying with methanol. (d) Photographs of the compound with caesiumcation particles on filter paper (diameter 110 mm) under UV irradiation (365 nm) after spraying with methanol.
Radioactive caesium 137 has a half-life of 30.17 years, and its accumulation in organisms in exposed regions, such as around the Fukushima Daiichi nuclear plant, amplifies the hazard it poses. A new material reported by researchers in Japan may help. "We have developed molecular materials as an optical probe for caesium cation-containing particles with implementation based on simple spray-on reagents and a commonly available fluorescent lamp for naked-eye detection in the solid state," explain Jonathan P Hill, Katsuhiko Ariga and colleagues at the WPI-International Center for Materials Nanoarchitectonics, Tokyo University and the Japan Science and Technology Agency (JST).
The researchers based the probe on changes in the fluorescence of organic compounds called phenols in the presence of alkali metals. They designed a substituted phenol compound with an electron-accepting 4-nitrophenyl ether (4-NPE) group. The compound showed a distinctive green fluorescence in the presence of caesium cations and methanol. In comparison, other alkali metals gave rise to dimmer blue fluorescence.
The researchers noted that the fluorescence peak shifted with increasing caesium concentration so that the probe could be calibrated. The probe was sensitive to caesium concentrations of 1 part per million. This is the concentration naturally occurring in the Earth's crust meaning excess to the norm can be detected.
Alongside molecular dynamics calculations these observations suggested the mechanism behind the fluorescent behaviour, for which the geometry of the compound molecules and interactions between different constituents seem key.
As the authors suggest, "The availability of this and other similarly easily implemented tests for environmental contaminants is likely to increase the volume of data regarding rates of contamination around chemical and radiological hazards."
"Micrometer-level naked-eye detection of caesium particulates in the solid state"
Taizo Mori, Masaaki Akamatsu, Ken Okamoto, Masato Sumita,Yoshitaka Tateyama, Hideki Sakai,
Jonathan P Hill, Masahiko Abe, and
Katsuhiko Ariga
Journal : Sci. Technol. Adv. Mater. [7 February 2013]
DOI :
10.1088/1468-6996/14/1/015002
International Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS), Namiki 1-1, Tsukuba, Ibaraki 305-0044, Japan
References
"Micrometer-level naked-eye detection of caesium particulates in the solid state", Taizo Mori, Masaaki Akamatsu, Ken Okamoto, Masato Sumita,Yoshitaka Tateyama, Hideki Sakai, Jonathan P Hill, Masahiko Abe, Katsuhiko Ariga, 2013 Sci. Technol. Adv. Mater, 14 015002
ナノアーキテクトニクス材料研究センター(MANA)
〒305-0044 茨城県つくば市並木1-1
TEL: 029-860-4710
E-mail: mana-pr=ml.nims.go.jp([ = ] → [ @ ] )
To receive our e-mail newsletter “MANA Research Highlights”, please send an e-mail with "MANA Research Highlights request” in the subject line or main text to the following address: mana-pr_at_ml.nims.go.jp
*Please change "_at_ " in the email address to @.
 Research Highlights
Research Highlights