- ホーム
- > 研究活動
- > Research Highlights
- > Vol. 37 Metal Oxide/Graphene Nanosheet・・・
 Research Highlights
Research Highlights
[Vol. 37]
Metal Oxide/Graphene Nanosheet Composite Exhibits Unprecedented Energy Storage Properties
- 前の記事
- 一覧に戻る
- 次の記事
2018.03.29
Single-layer metal oxide nanosheets sandwiched between graphene layers exhibit record energy storage figures of merit.
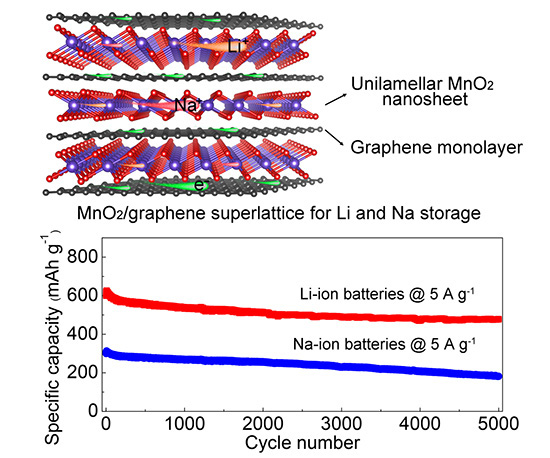
Figure: Structure of MnO2/graphene superlattice-like structure and electrochemical characteristics of Li and Na batteries fabricated using this material.
The unique physical properties of nanomaterials are important for the realization of high performance energy storage devices. For example, the ultra-narrow spaces between multilayered two-dimensional (2D) nanosheets are suitable for high efficiency ion intercalation, namely, reversible insertion of ions into the spaces between layers. Importantly, nanomaterial structures with nearly ideal nano interlayer spaces for ion intercalation exhibit short diffusion distances and large numbers of active sites —properties that are essential for fabricating electrode materials of high performing energy storage devices.
However, despite extensive research on 2D materials such as graphene and transition metal dichalcogenides the energy storage capacity has not met expectations. More recently, research has also turned to explore 2D metal oxides—materials with many more active sites, and even shorter diffusion lengths. The main issues to resolve include synthesis of genuine single layered metal oxides, prevention of ‘restacking’ during chemical processes to fabricate electrodes, and improving their electrical conductivity.
Here, a group led by Takayoshi Sasaki at the International Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS), Tsukuba, Japan, describe the synthesis of superlattice-like MnO2/graphene 2D nanostructures that exhibited the best figures of merit for energy storage reported to-date.
The researchers synthesized the MnO2/graphene superlattice structures by ‘electrostatic assembly’ of MnO2 and graphene in a solution, exploiting the differences in the charged states of the respective materials: The MnO2 nanosheets are negatively charged and reduced graphene oxide (rGO) nanosheets are positive. The two important points about this fabrication process and the resulting nanosheet structures are that the MnO2 nanosheets were ‘genuine unilamellar’ structures and each of the MnO2 nanosheets was ‘stabilized’ between the atomic layers of graphene.
The MnO2/graphene nanostructures were used as anodes for Li and Na ion batteries. Electrochemical measurements showed specific capacities of 1325 and 795 mA h g−1 at 0.1 A g−1 and 370 and 245 mA h g−1 at 12.8 A g−1 for Li and Na storage, respectively. “More importantly, an ultralong cyclability with 0.004% and 0.0078% capacity decay per cycle up to 5000 cycles was achieved for Li and Na storage, respectively, outperforming previously reported metal oxide-based anodes to date,” state the authors.
“The superlattice composite material described in this paper is based on MANA’s concept of ‘Materials Nanoarchitectonics’, says Sasaki. “We have taken two types of 2D materials with differing properties and formed an advanced composite material with synergistic characteristics that are not exhibited by a single material.”
Reference
“Genuine Unilamellar Metal Oxide Nanosheets Confined in a Superlattice-like Structure for Superior Energy Storage”
Pan Xiong, Renzhi Ma, Nobuyuki Sakai, and Takayoshi Sasaki
Journal : ACS Nano, 12 ,1768–1777, (2018).
DOI : 10.1021/acsnano.7b08522
Pan Xiong, Renzhi Ma, Nobuyuki Sakai, and Takayoshi Sasaki
Journal : ACS Nano, 12 ,1768–1777, (2018).
DOI : 10.1021/acsnano.7b08522
Affiliations
International Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS), Namiki 1-1, Tsukuba, Ibaraki 305-0044, Japan
Contact information
ナノアーキテクトニクス材料研究センター (MANA)
〒305-0044 茨城県つくば市並木1-1
TEL: 029-860-4710
E-mail: mana-pr=ml.nims.go.jp([ = ] → [ @ ] )
TEL: 029-860-4710
E-mail: mana-pr=ml.nims.go.jp([ = ] → [ @ ] )