Research Topics: On-Surface Chemistry with High-Resolution STM/AFM

We study molecules adsorbed on surfaces at the atomic scale using scanning tunneling microscopy (STM) and atomic force microscopy (AFM) under ultra-high vacuum conditions at low temperatures as our main measurement techniques. By terminating the tip apex of STM or AFM with a small molecule or noble gas atom, it has become possible to directly visualize the inner structures of molecules adsorbed on surfaces in real space. This advanced measurement technology represents a major breakthrough in the long history of chemistry. For example, we can directly observe the structure of a precursor molecule, then induce a reaction by flowing a tunneling current at a given bias voltage between the tip and the smaple, thereby altering its structure, and subsequently identify the structure of the resulting product in situ. This method enables the synthesis of novel compounds that are difficult to achieve through conventional solution-based synthetic chemistry. It is a truly promising research field with the potential to become a new branch of chemistry. Novel carbon compounds, such as graphene nanoribbons synthesized through thermally induced chemical reactions, are also expected to be developed as materials for nanoelectronics. Furthermore, recent advances have enabled the synthesis of molecules with unpaired electrons, opening up the field of π-electron spin engineering with carbon nanostructures on surfaces.
Besides exploring the basic science, tip-induced and/or thermal on-surface reaction lead to syntheses of novel nanocarbon material such as graphene nanoribbons, which are expected to be a key element in nano-electronics and spintronics. For instance, various applications such as gas sensor, magnetic head, light emitter/detected, switch, electrostatic capacitor, superlubricity interface, and spintronics can be considered. We wish that our scientific research with STM/AFM develops on-surface chemistry and consequently realizes nanocarbon based devices. We are also developing new instruments and measurement techniques, which are of importance to proceed our studies.
Summary of our study
Previous Achievement

1. Properties of Single Molecules
Aromaticity of single molecules was investigated via direct observations of molecular structures with a carbon monoxide (CO) molecule terminated tip of STM/AFM. We studied the bond orders in the molecules, having four- and eight-membered rings at their centers, by measuring the apparent C-C bond lengths (Fig. 1A and 1B) and found modification of anti-aromatic property at a single molecular level.
1,2 With heterocyclic compounds with boron and/or nitrogen atoms, the six-membered rings were strongly distorted in the bond-resolved images due to the modulated total charge density of C-B and C-N bonds (Fig. 1C).
3 In this way, the bond-resolved imaging allows the determination of the aromatic properties via the direct measurement of the apparent bond lengths.
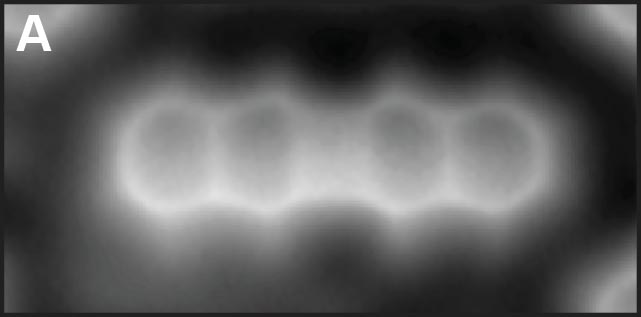
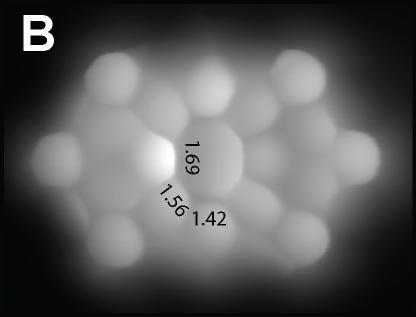
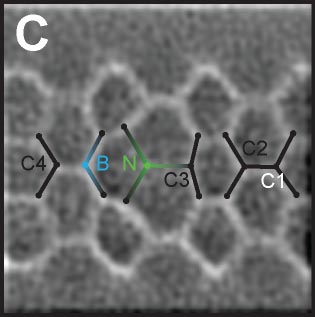
Fig. 1(A) Dibenzo[b,h]biphenylene.1 (B) π-extended dDiaza[8]circulene.2 (C) C4BN six-membered ring in graphene nanoribbon.3
--Related articles--
[1] S. Kawai, K. Takahashi, S. Ito, R. Pawlak, T. Meier, P. Spijker, F. Federici Canova, J. Tracey, K. Nozaki, A. S. Foster, E. Meyer. ACS Nano 2017, 11, 8122-8130.

[2] K. Nakamura, Q.-Q. Li, O. Krejci, A. S. Foster, K. Sun, S. Kawai, S. Ito. J. Am Chem. Soc. 2020, 142, 11363. 
[3] S. Kawai, S. Nakatsuka, T. Hatakeyama, R. Pawlak, T, Meier, J. Tracey, E. Meyer, A. S. Foster. Science Advances 2018, 4, eaar7181. 
2. Self-Assembled Molecular Structure
Using a tip terminated with CO molecule, we directly investigated the chemical structure of the self-assembled molecular films condensed by the weak C-F···H-C hydrogen bonding (Fig. 2A).4 Although there are many similar nanoscale studies using STM, this result was the first example to experimentally determine the self-assembled molecular structure with atomic-level precision. We also found that fluorinated molecules can form structures on Ag(111) similar to conventional halogen bonds, ma,e;u tje extemded ja;pgem bpmdomg (Fig. 2B).5 Furthermore, two different complex nanoporous molecular networks were obtained by replacing the central thiophen core in the dibrominated molecule by furan core. The structures allowed precise engineering of quantum dot array coupling through their barrier widths (Fig. 2C).6 By precisely designing halogen bonds, we succeeded in creating self-assembled films with three different sizes of vacancies, and in controlling the surface states using these vacancies (Figure 2D).7
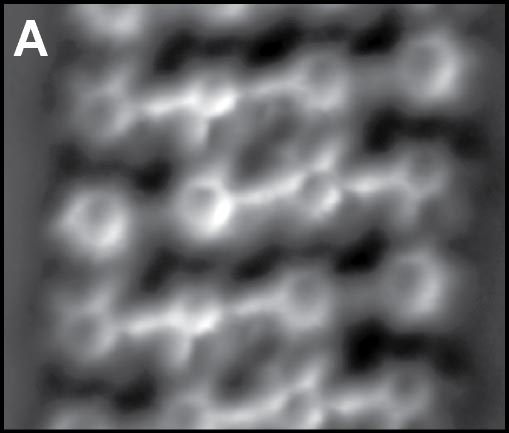
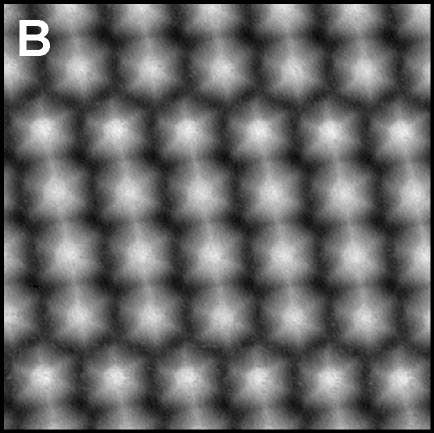
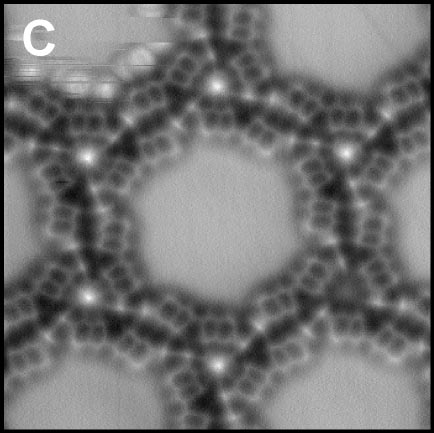
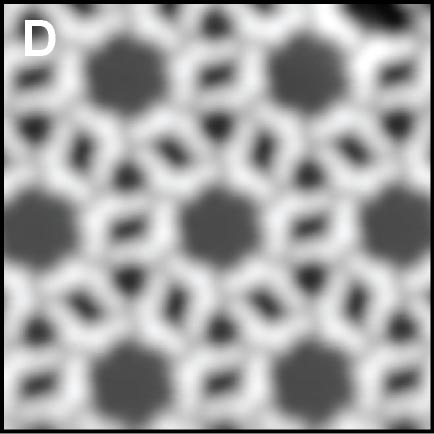
Fig. 2(A) C-F···H weak hydrogen bonding.4 (B) C-F···F extended halogen bonding.5 (C) C-Br···Br/O multiple-halogen bonding.6 (D) C-Br···Br/N hetero-halogen bondings.7
--Related articles--
[4] S. Kawai, A. Sadeghi, X. Feng, P. Lifen, R. Pawlak, T. Glatzel, A. Willand, A. Orita, J. Otera, S. Goedecker, E. Meyer. ACS Nano 2013, 7, 9098. 
[5] S. Kawai, A. Sadeghi, F. Xu, L. Peng, A. Orita, J. Otera, S. Goedecker, E. Meyer. ACS Nano 2015, 9, 2574. 
[6] I. Piquero-Zulaica, J. Lobo-Checa, A. Sadeghi, Z. M. Abd El-Fattah, C. Mitsui, T. Okamoto, R. Pawlak, T. Meier, A. Arnau, J. E. Ortega, J. Takeya, S. Goedecker, E. Meyer, S. Kawai. Nature Commun. 2017, 8, 787. 
[7] S. Kawai, M. A. Kher-Elden, A. Sadeghi, Z. M. Abd El-Fattah, K. Sun, S. Izumi, S. Minakata, Y. Takeda, J. Lobo-Checa. Nano Lett. 2021, 21, 6456. 
3. Atomic and Intermolecular Forces
The van der Waal force between atoms is one of the most important and fundamental interactions in chemistry and physics. We demonstrated a quantitative force measurement by anchoring closed-shell rare gas atoms (Ar, Kr, and Xe) at specific sites of metal organic framework (MOF) networks, and sensing them with the Xe terminated AFM tip (Fig. 3A).8 Furthermore, a quantitative force measurement of the hydrogen bonding between the CO terminated tip and the three dimensional hydrocarbon adsorbed on surface was also demonstrated (Fig. 3B).9 In the measurement, we found that the CO terminated tip can resolve positions of hydrogen atoms in molecules (Fig. 3C). This method allows direct structural analyses of three-dimensional molecules such as DNA.
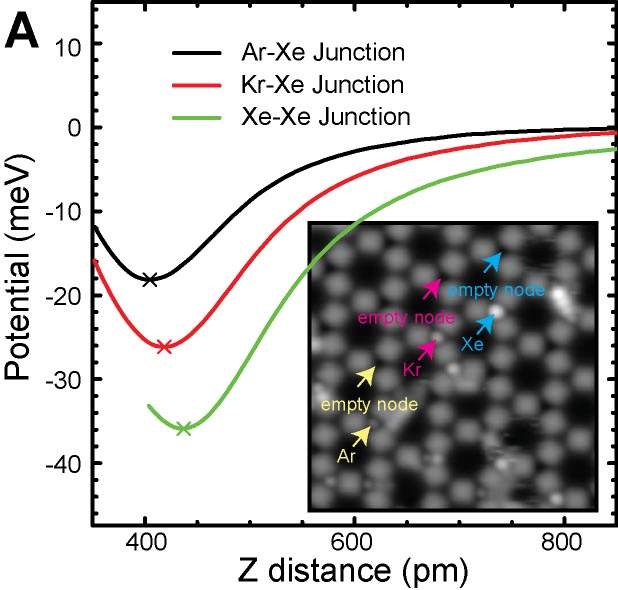
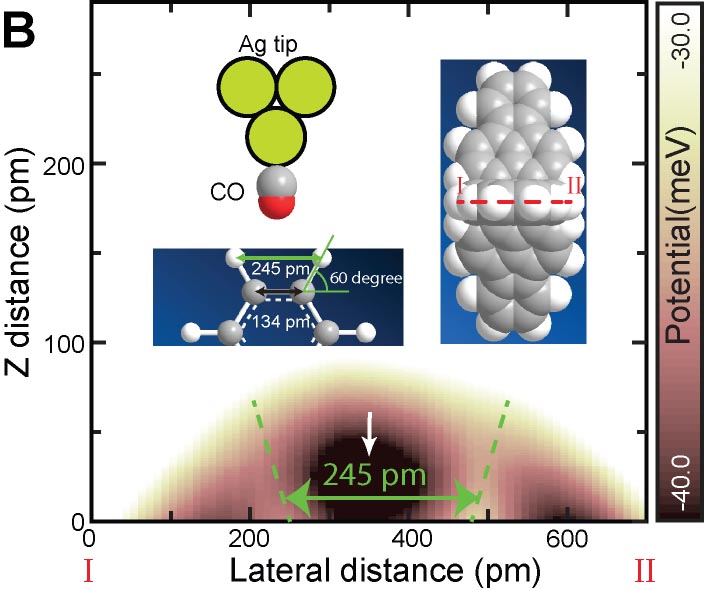
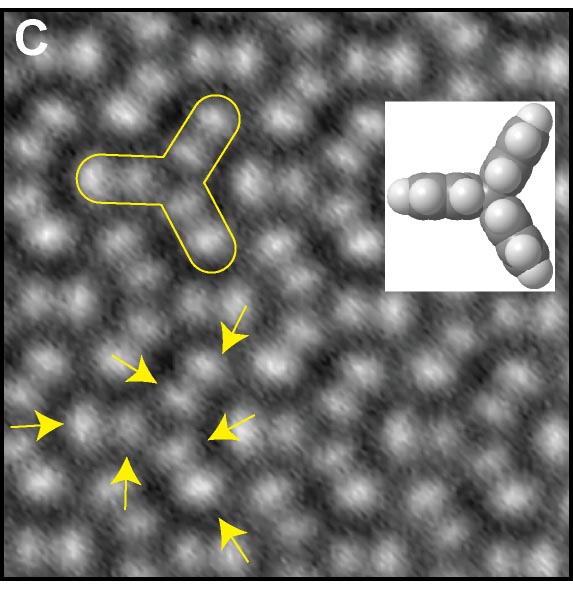
Fig. 3(A) Quantitative force measurements of atomic van der Waals interaction8 and (B) hydrogen bonding. (C) Visualization of hydrogen atoms in three dimensional hydrocarbon.9
--Related articles--
[8] S. Kawai, A. S. Foster, T. Björkman, S. Nowakowsaka, J. Björk, F. Federici Canova, L. H. Gade, T. A. Jung, E. Meyer. Nature Commun. 2016, 7, 11559. 
[9]
S. Kawai, T. Nishiuchi, T. Kodama, P. Spijker, R. Pawlak, T. Meier, J. Tracey, T. Kubo, E. Meyer A. S. Foster. Science Advances 2017, 3, e1603258. 
4. On-Surface Chemical Reaction
We demonstrated the structural analysis of precursor, intermediates, and product in three-step thermal transformation of the acetylene groups in a single molecule (Fig. 4A).10 This study shows that high-resolution observation by AFM and STM is extremely valuable for analyzing products. On-surface chemical reactions are an important technique for bottom-up synthesis of carbon nanostructures. Using this reaction, we have achieved homo-coupling between silyl groups, which are commonly used as protecting groups in organic synthesis (Fig. 4B),11 directional hetero-coupling between chloro and silyl groups (Fig. 4C),12 as well as homo-coupling of -CF3 groups (Fig. 4D)13 and methylene groups for the synthesis of block co-oligomers (Fig. 4E).14 Additionally, we found that depositing silicon on the surface beforehand enables the formation of C-Si-C bonds during dehalogenation. Using this reaction, we successfully synthesized two-dimensional covalent organic frameworks (COFs) containing silicon (Fig. 4F).15 In addition to research on linking small molecules to synthesize larger structures, we have developed reactions that cleave C–C single bonds via Diels–Alder reactions (Fig. 4G).16
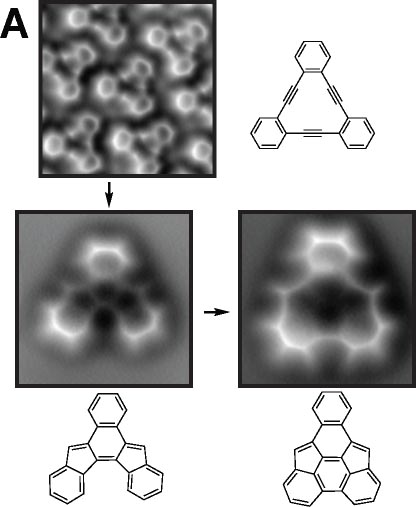
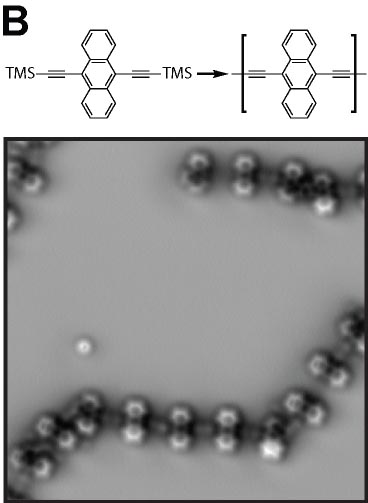
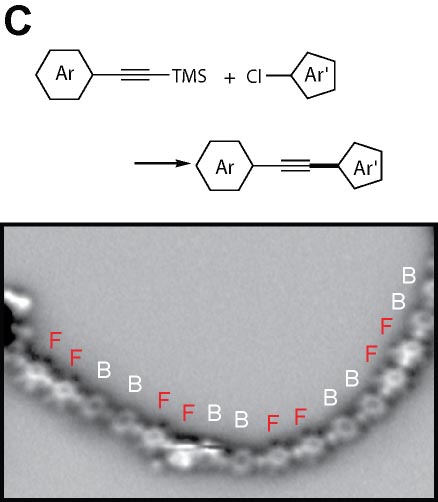
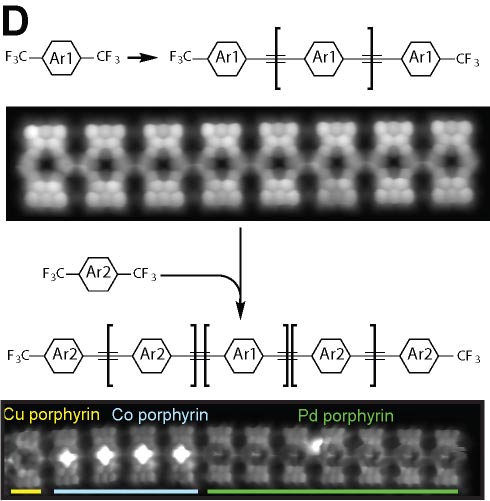
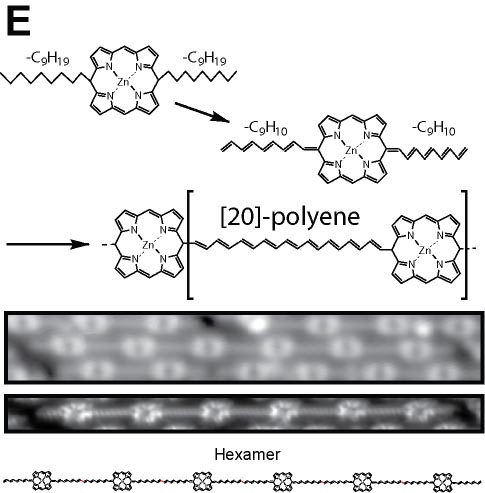
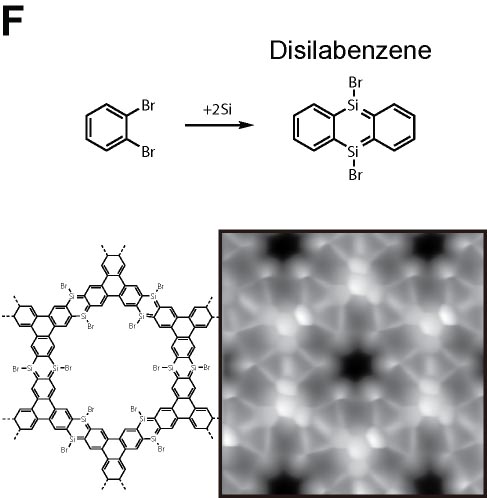
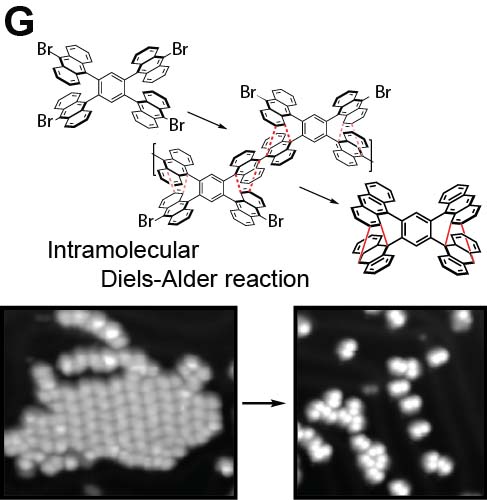
Fig. 4(A) Structural analysis of sequential on-surface reaction.10. (B) Homo-coupling between TMS groups.11 (C) Hetero-coupling between TMS and -Cl groups.12 (D) Surface synthesis of block co-oligomers via homo-coupling of CF3 groups13 and (E) methylene groups.14 (F) On-surface synthesis of COF films containging disilabenzene as a linker.15 (G) Cleavage of C–C single bonds via Diels–Alder reactions.16
--Related articles--
[10] S. Kawai, V. Haapasilta, B. D. Lindner, K. Tahara, P. Spijker, J. A. Buitendijk, R. Pawlak, T. Meier, Y. Tobe, A. S. Foster, E. Meyer. Nature Commun. 2016, 7, 12711. 
[11] S. Kawai, O. Krejčí, A. S. Foster, R. Pawlak, F. Xu, L. Peng, A. Orita, E. Meyer. ACS Nano 2018, 12, 8791. 
[12] K. Sun, K. Sagisaka, L. Peng, H. Watanabe, F. Xu, R. Pawlak, E. Meyer, Y. Okuda, A. Orita, S. Kawai. Angew. Chem. Int. Ed. 2021, 60, 19598. 
[13] S. Kawai, A. Ishikawa, S. Ishida, T. Yamakado, Y. Ma, K. Sun, Y. Tateyama, R. Pawlak, E. Meyer, S. Saito, A. Osuka. Angew. Chem. Int. Ed. 2022, 61, e202114697. 
[14] K. Sun, A. Ishikawa, R. Itaya, Y. Toichi, T. Yamakado, A. Osuka, T. Tanaka, K. Sakamoto, S. Kawai. ACS Nano 2024, 18, 13551. 
[15] K. Sun, O. J. Silveira, Y. Ma, Y. Hasegawa, M. Matsumoto, S. Kera, O. Krejčí, A. S. Foster, S. Kawai. Nature Chemistry 2023, 15, 136.
[16] D. Li, T. Ohto, T. Nishiuchi, S. Takeuchi, Y. Nishide, H. Kimizuka, T. Kubo, S. Kawai. ACS Nano 2025, 19, 35825. 
5. Graphene Nanoribbons
On-surface reaction allows systematic syntheses of graphene nanoribbons (GNRs) with controlled edge structures and widths. We presented GNRs, substituted by boron atoms at the specific sites (Fig. 5A).17 Additionally, we have also achieved the synthesis of GNRs doped with both boron and nitrogen.18 By introducing five-membered or seven-membered rings at the GNR terminus, we succeeded in synthesizing GNRs with nonplanar structures (Fig. 5B).19 Furthermore, we have also succeeded in synthesizing three-dimensional GNRs using propeller-shaped precursor molecules (Fig. 5C).20 Employing a newly developed on-surface reaction to introduce silicon, we have successfully synthesized GNRs containing silicon (Fig. 5D).21
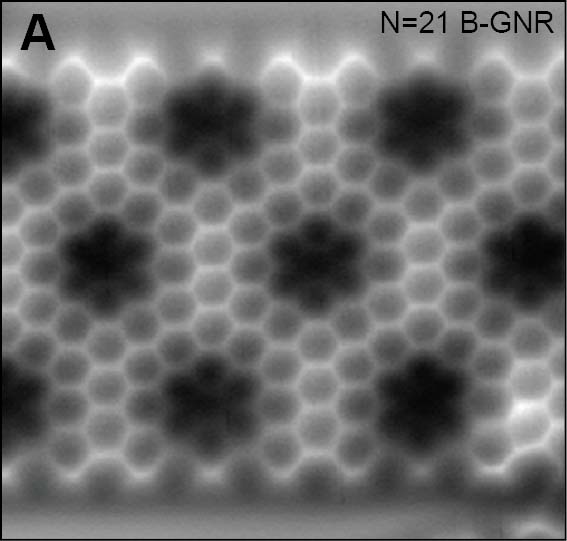
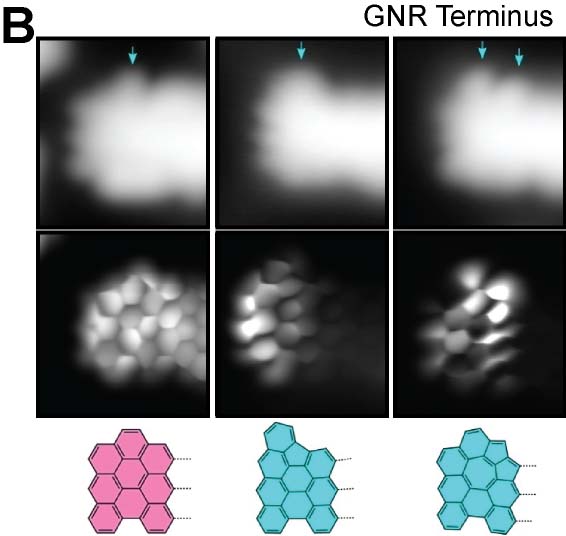
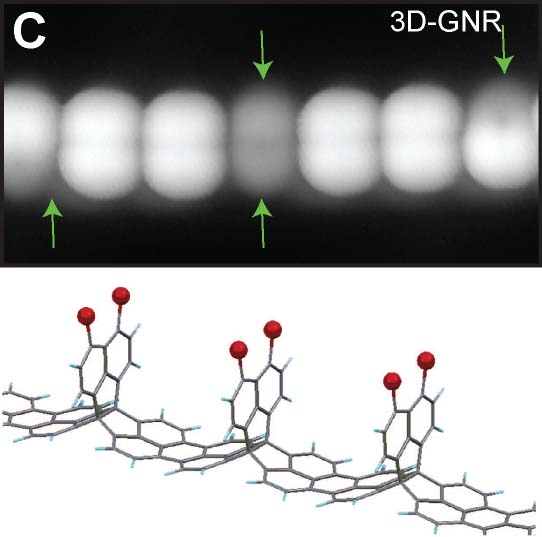
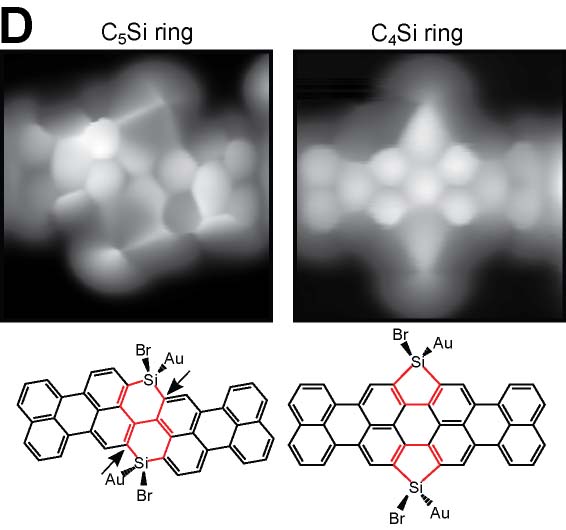
Fig. 5(A) Boron-doped graphene nanoribbon.17 (B) GNR with nonplanar terminus containg five-membered and seven-membered rings.19 (C) Three-dimensional GNR.20 (D) Silicon-doped GNR.21
--Related articles--
[17] S. Kawai, S. Saito, S. Osumi, S. Yamaguchi, A. S. Foster, P. Spijker, E. Meyer. Nature Commun. 2015, 6, 8098. 
[18] S. Kawai, S. Nakatsuka, T. Hatakeyama, R. Pawlak, T, Meier, J. Tracey, E. Meyer, A. S. Foster. Science Advances 2018, 4, eaar7181. 
[19] X. Xu, K. Sun, A. Ishikawa, A. Narita, S. Kawai. Angew. Chem. Int. Ed. 2023, 62, e202302534. 
[20] S. Kawai, O. Krejčí, T. Nishiuchi, K. Sahara, T. Kodama, R. Pawlak, E. Meyer, T. Kubo, A. S. Foster. Science Advances 2020, 6, eaay8913. 
[21] K. Sun, L. Kurki, O. J. Silveira, T. Nishiuchi, T. Kubo, A. S. Foster, S. Kawai. Angew. Chem. Int. Ed. 2024, 63, e202401027. 
6. Tip-Induced Local Chemistry
By removing a halogen atom from a molecule by tip, we can synthesize highly reactive radical species. Such the radical is usually stablized by connecting to the surfac emetal atom. However, by conducting the dissociation reaction on thin insulating films such as NaCl, it is possible to generate long-lived radicals. Repeating this dissociation reaction allows for the synthesis of even more reactive diradicals and, ultimately, successful structural isomerization (Fig. 6A).22 In addition to dissociation reactions of the planar molecules, we have also succeeded in dissociating bromine atoms protruding from three-dimensional graphene nanoribbons. Furthermore, we achieved an addition reaction in which a bromine atom adsorbed onto the tip apex was bonded to the radical site generated by the dissociation reaction. Beyond single atoms, we also realized addition reactions that directly link different molecules, such as fullerene molecules (Fig. 6B).23 Detailed studies of the sites where bromine was dissociated revealed that they can adopt three different structures: two dehydroazulenes and a diradical. Flowing a tunneling current, we successfully induced highly reproducible structural isomerization among these three states (Fig. 6C).24
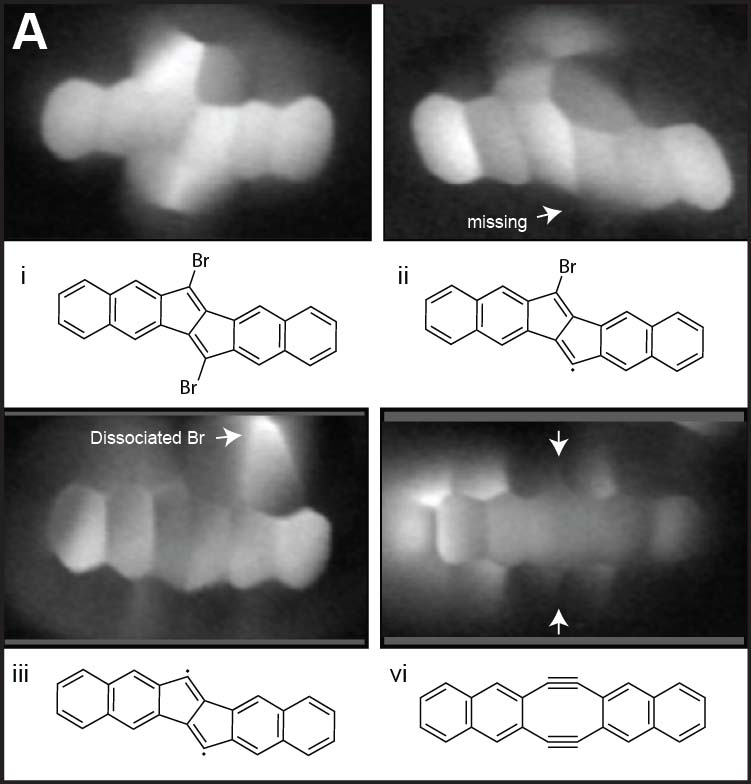
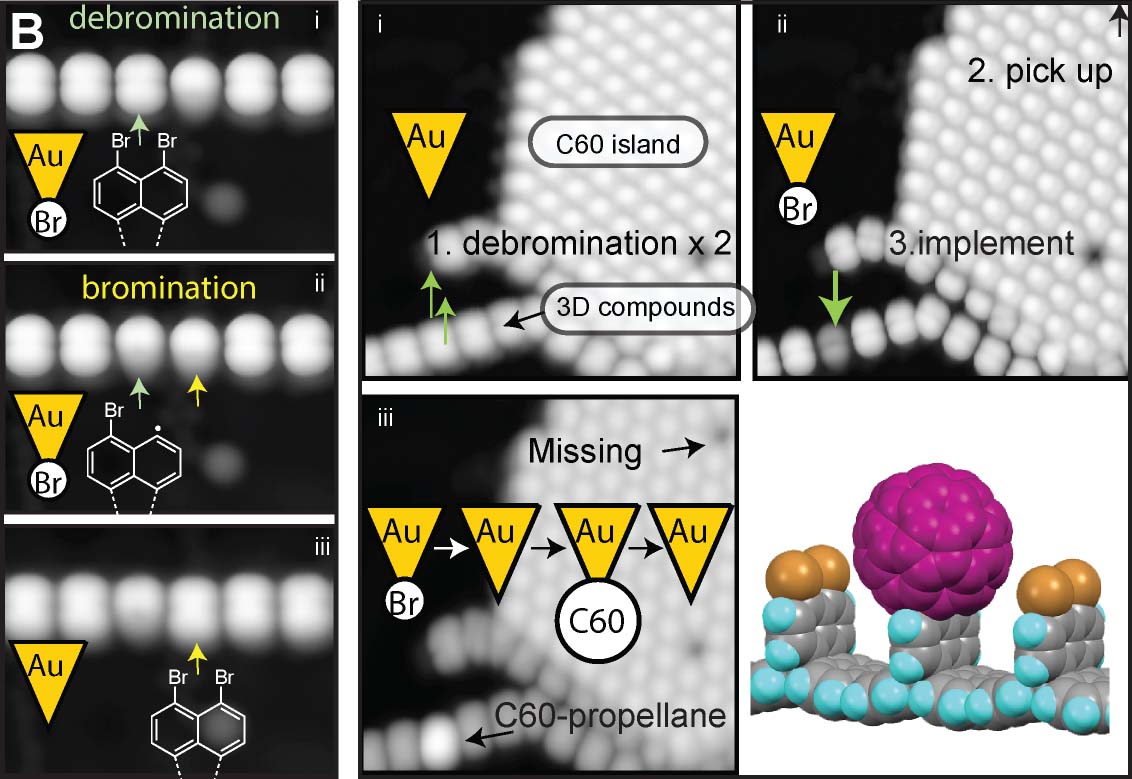
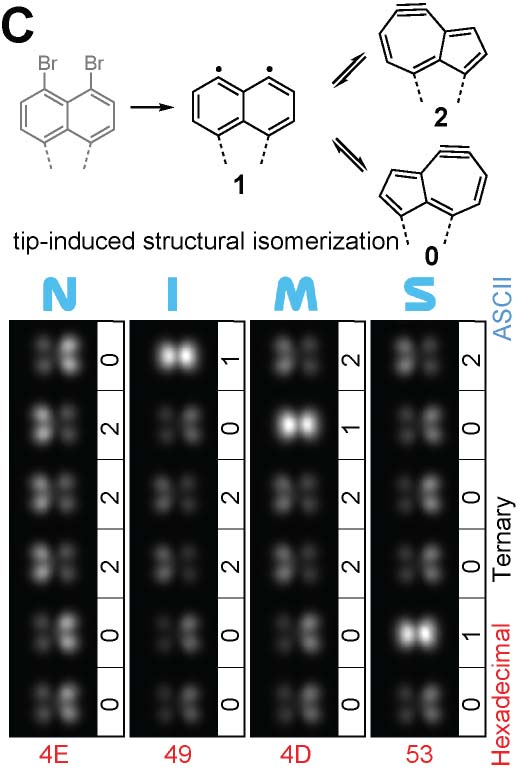
Fig.6(A) Tip-induced debromination and structural isomerization of a planar molecule.22 (B) Debromination and bromination in the three-dimensional GNRs, and addition reaction between the GNR and a fullerene molecule.23 (C) Structural isomerization among two dehydroazulenes and a diradical.24
--Related articles--
[22] S. Kawai, H. Sang, L. Kantorovich, K. Takahashi, K. Nozaki, S. Ito. Angew. Chem. Int. Ed. 2020, 59, 10842. 
[23] S. Kawai, O. Krejčí, T. Nishiuchi, K. Sahara, T. Kodama, R. Pawlak, E. Meyer, T. Kubo, A. S. Foster. Science Advances 2020, 6, eaay8913. 
[24] S. Kawai, O. J. Silveira, L. Kurki, Z. Yuan, T. Nishiuchi, T. Kodama, K. Sun, O. Custance, J. L. Lado, T. Kubo, A. S. Foster. Nature Communications 2023, 14, 7741. 
7. Mechanical Properties of Nanocarbon Structures
It was challenging to measure the mechanical property of a given molecular strand. We demonstrated precise measurement of single oligomer synthesized by on-surface reaction, by picking up with an AFM tip (Fig. 7A).25 This method allowed determination of the adsorption energy of each unit. Furthermore, we investigated the mechanism of structural superlubricity at the contact between the graphene nanoribbon (GNR) and the underlying Au(111) surface by dragging the GNR by the tip (Fig. 7B).26.
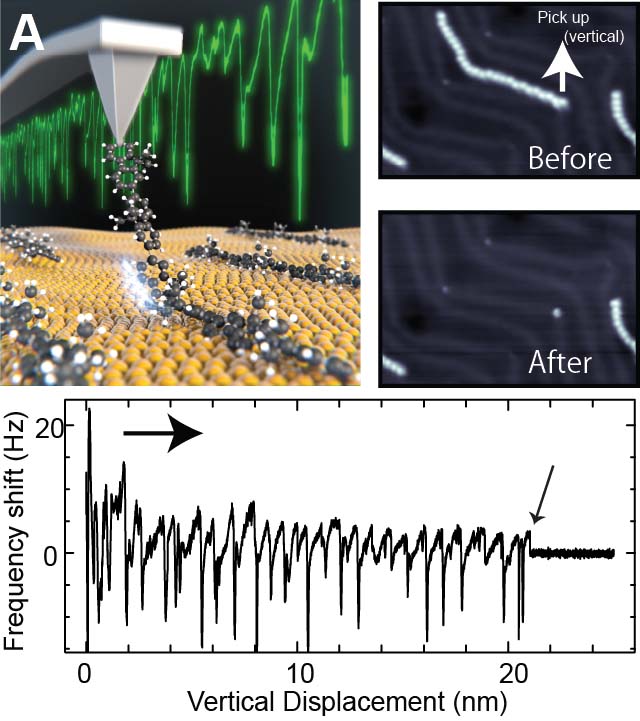
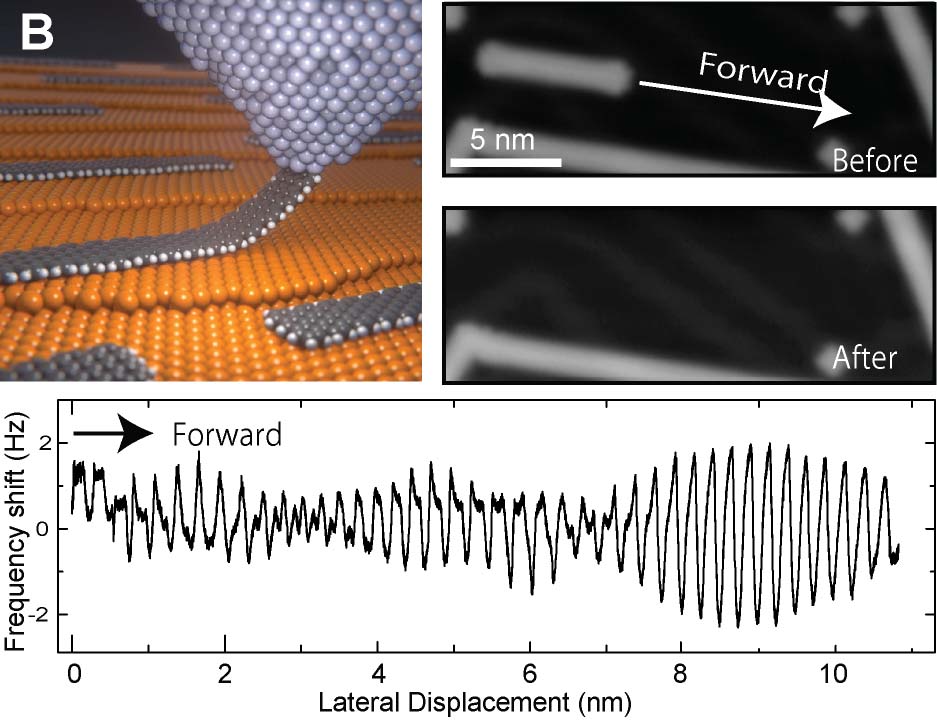
Fig. 7(A) Lifting measurement of a single oligomer.25 (B) Structural superlubricity with GNR.26
--Related articles--
[25] S. Kawai, M. Koch, E. Gnecco, A. Sadeghi, R. Pawlak, T. Glatzel, J. Schwarz, S. Goedecker, S. Hecht, A. Baratoff, L. Grill, E. Meyer. Proc. Natl. Acad. Sci. USA 2014, 111, 3968. 
[26] S. Kawai, A. Benassi, E. Gnecco, H. Söde, R. Pawlak, K. Mullen, D. Passerone, C. Pignedoli, P. Ruffieux, R. Fasel, E. Meyer. Science 2016, 351, 957. 
8. Magnetic Properties of Nanocarbon Structures
We have succeeded in measuring the magnetism of carbon nanostructures synthesized on surfaces. By introducing boron at specific sites in graphene nanoribbons, spin states can be generated. Normally, these spin states disappear on metal substrates, but we found that they can be maintained on AuSix intercalation films, which act as electrical intercalation layers. Furthermore, we were able to remove silicon atoms adsorbed at boron sites during the formation of the intercalation film, and found that the spin states transfer in a relay-like manner (Fig. 8A).27 For single molecules possessing spin, we achieved the synthesis of nitrogen-doped [5]triangulene (Fig. 8B),28 control of spin states through bonding between neutral triangulene molecules and silicon in the AuSix intercalation film (Fig. 8C),29 and manipulation of the spin states of nickelocene and cobaltocene molecules by connecting to bromine (Fig. 8D).30 Additionally, we have realized the synthesis of one-dimensional spin-1/2 Heisenberg molecular chains (Fig. 8E)31 and rings (Fig. 8F).32
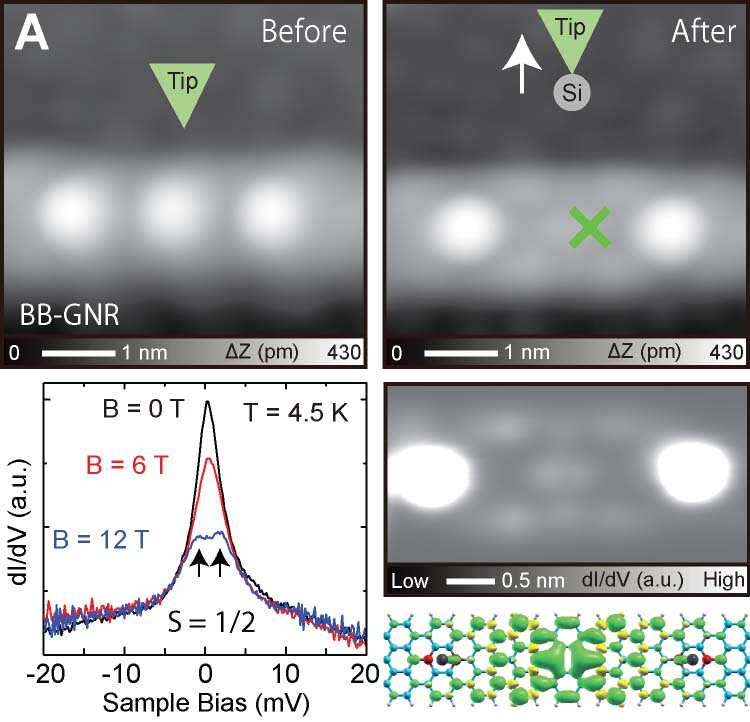
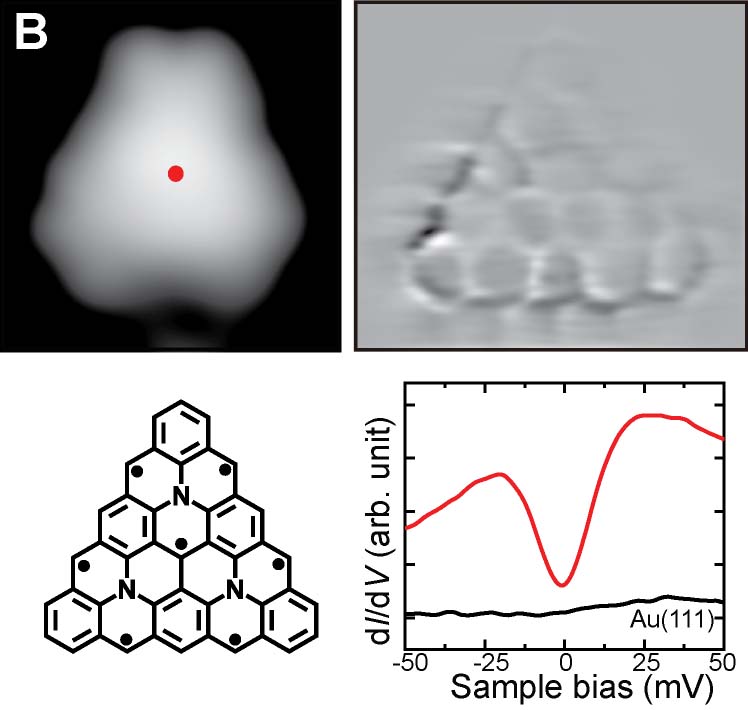
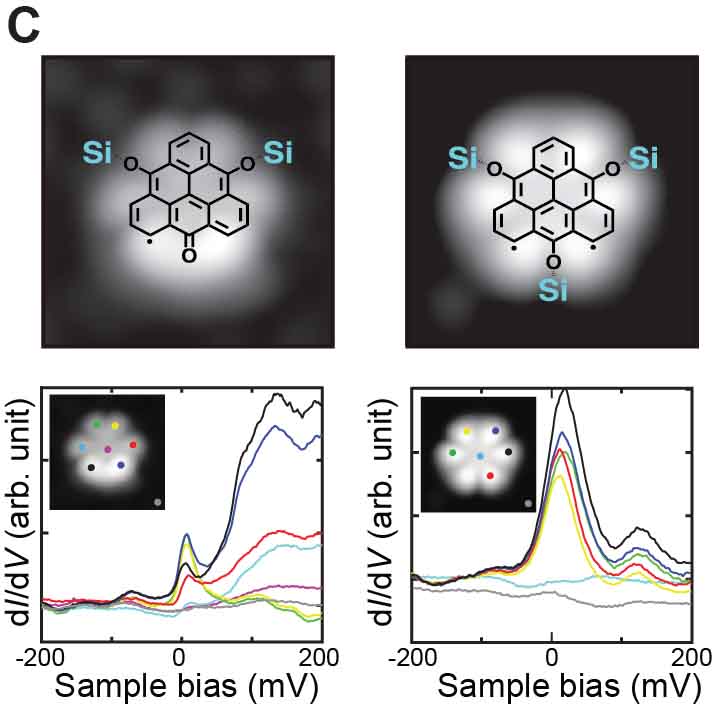
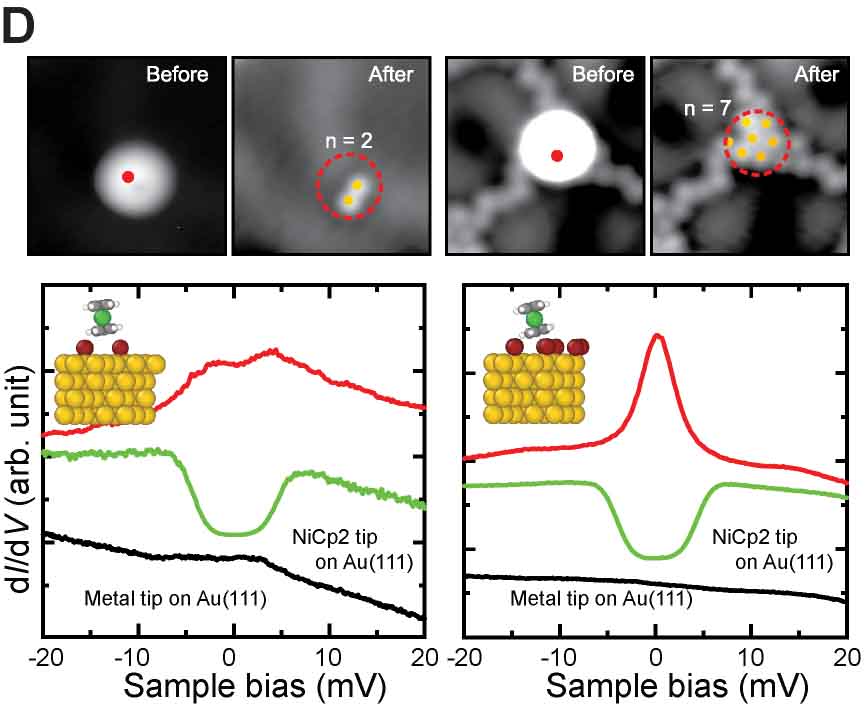
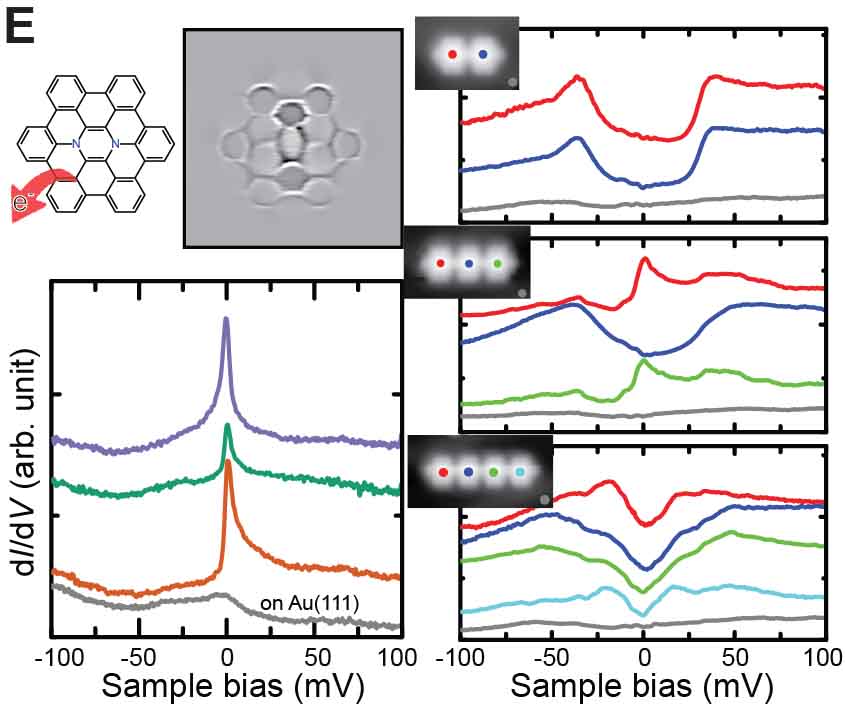
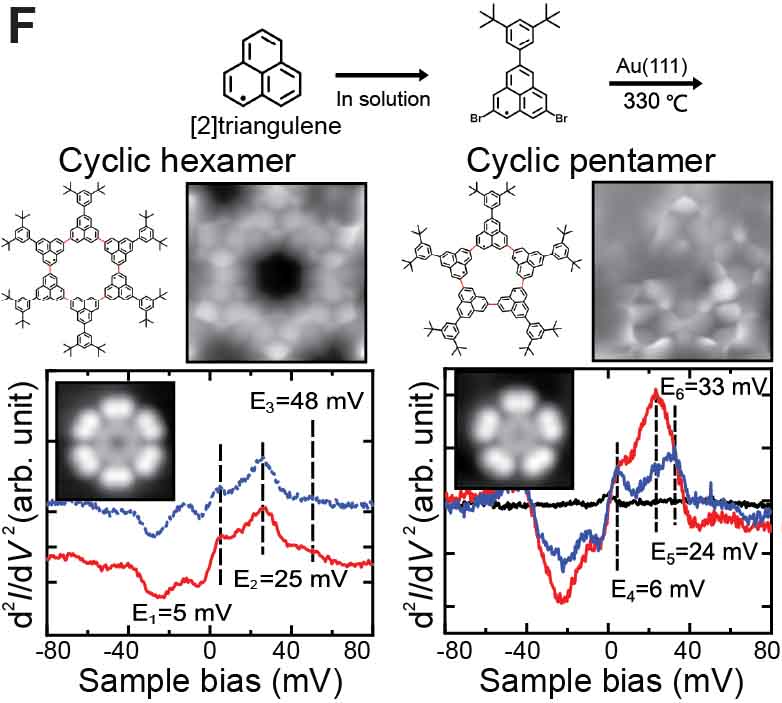
Fig. 8(A) Spin measurement in boron-doped GNRs.27 (B) On-surface synthesis and spin measurement of [5]triangulene with three nitrogen atoms.28 (C) Control of spin state by Si-O bond formation in neutral triangulene molecules to silicon on the surface.29 (D) Control of the spin states of nickelocene and cobaltocene molecules by cpmmectomg to bromine.30 (E) On-surface synthesis and spin states of one-dimensional spin-1/2 Heisenberg molecular chains.31 (F) On-surface synthesis and spin states of ring-shaped spin-1/2 assemblies.32
--Related articles--
[27] K. Sun, O. J. Silveira, S. Saito, K. Sagisaka, S. Yamaguchi, A. S. Foster, S. Kawai. ACS Nano 2022, 16, 11244. 
[28] D. Li, O. J. Silveira, T. Matsuda, H. Hayashi, H. Maeda, A. S. Foster, S. Kawai. Angew. Chem. Int. Ed. 2024, 63, e202411893. 
[29] Z. Yuan, T. Kariyado, T. Murata, K. Sun, D. Lin, O. Custance, Y. Morita, S. Kawai. Nano Letters 2025, 25, 13040. 
[30] D. Li, N. Cao, A. S. Foster, S. Kawai. J. Am. Chem. Soc. 2026, 148, 3356–3364. 
[31] K. Sun, N. Cao, O. J. Silveira, A. O. Fumega, F. Hanindita, S. Ito, J. L. Lado, P. Liljeroth, A. S. Foster, S. Kawai.Science Advances 2025, 11, eads1641. 
[32] D. Li, N. Cao, M. Metzelaars, O. Silveira, J. Jestilä, A. Fumega, T. Nishiuchi, J. Lado, A. S. Foster, T. Kubo, S. Kawai. J. Am. Chem. Soc. 2025, 147, 26208. 
9. Bimodal AFM
Among several resonance modes of the AFM force sensor, conventional AFM usually uses the frequency shift of the first flexural mode to detect vertical tip-sample interaction forces. We proposed bimodal AFM, in which two resonance modes are simultaneously excited and detected, to realize higher sensitive measurement by small amplitude operation33 and multi-dimensional vector force measurement. For instance, vertical and lateral tip-sample interaction forces can be detected by frequency shifts of flexural and torsional oscillation modes, respectively (Fig. 9A).34 Since the lateral force on an atomically flat surface corresponds to only short-ranged atomic forces, atomically resolved imaging can routinely be obtained even with a non-ideal tip. Furthermore, this high-sensitive measurement realized atom manipulation on bulk insulating surface (NaCl), in which Br and Cl atoms were replaced by the AFM tip. (Fig. 9B).35 In a vicinity of the atomic step of the LiF(001) surface, the imbalanced electrostatic force, due to the sub-picometer shifts of anion and cation ions in the opposite directions by the applied bias voltage, was detected by the shifts of the torsional resonance (Fig. 9C).36
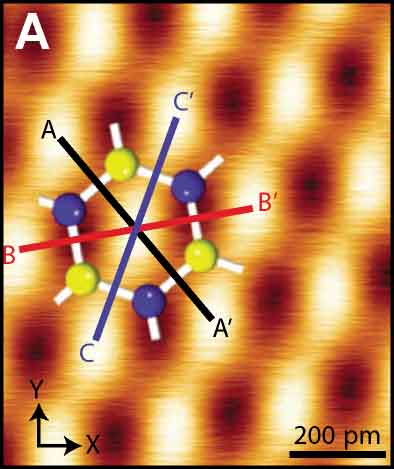
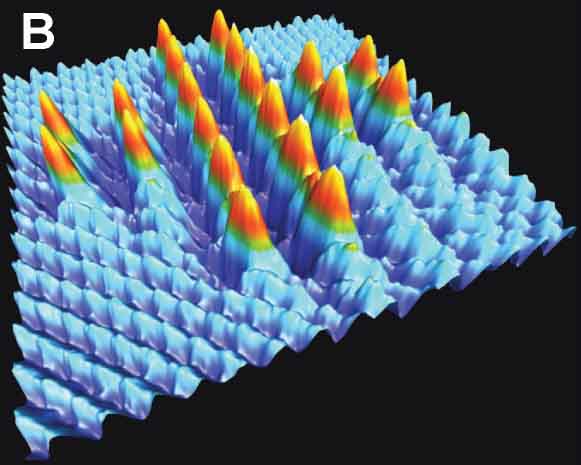
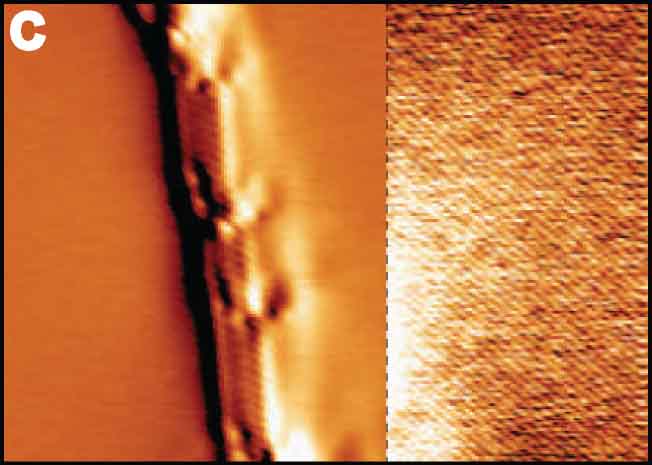
Fig. 9(A) Atomically resolved graphite surface by bimodal AFM at room temperature.34 (B) Atom manipulation on NaCl(001).35 (C) Modulated electrostatic force around the step of LiF(001).36
--Related articles--
[33] S. Kawai, T. Glatzel, S. Koch, B. Such, A. Baratoff, E. Meyer, Phys. Rev. Lett. 2009, 103, 220801. 
[34] S. Kawai, T. Glatzel, S. Koch, B. Such, A. Baratoff, E. Meyer, Phys. Rev. B. 2010, 81, 085420. 
[35] S. Kawai, A. S. Foster, F. F. Canova, H. Onodera, S. Kitamura, E. Meyer, Nature Commun. 2014, 5, 4403. 
[36] S. Kawai, F. F. Canova, T. Glatzel, T. Hynninen, E. Meyer, A. S. Foster, Phys. Rev. Lett. 2012, 109, 146101. 

















































