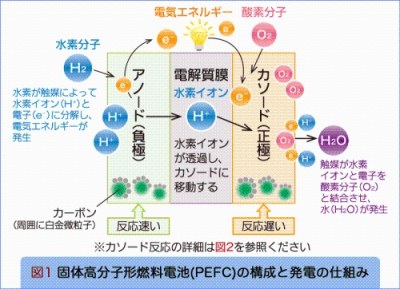
Both the positive and negative electrodes use catalysts in which a noble metal alloy containing platinum as a main component is supported on carbon black. It is necessary to overcome the following problems: Cost problems due to the scarcity and high price of platinum; internal resistance problems due to low positive electrode activity; and deterioration problems due to melting of platinum into an acidic solution and carbon monoxide (CO) poisoning of the negative electrode. Therefore, the electrode structure and the electrode reaction are clarified in detail from the simulation, and the research is carried out from two viewpoints of low platinum and diplatinum.
① Establishment of Prediction Method for Oxygen Reduction Activity Based on Reaction Energy Calculation
② Elucidation of Reaction Mechanism in Graphene-supported Monoatomic Electrodes by First-Principles Calculations
③ Analysis of Stable Structures and Electronic States of Monoatomic Catalysts in Doped Graphene