RESEARCH
7. Nanostructured Oxides/Chalcogenides
Transition metal oxides and metal chalcogenides are extremely important classes of compounds due to their electronic and optical properties, and also for their potential catalytic activities.
PbS, ZnS, CdS and Selenide Analogues
Chalcogenide nanomaterials are important electronic and optical nanomaterials. We are interested in how their forms and assembled states affect their properties. Thus, we have investigated their synthesis in collaboration with Prof. Somobrata Acharya (at the Indian Academy for the Cultivation of Science, Calcutta)
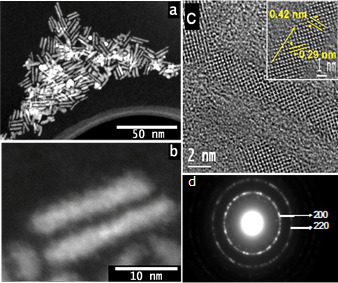
improve their contrast ratios and viewing angles.
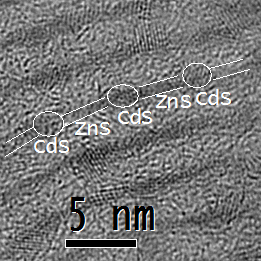
chemical programming of the starting materials.
Manganese Oxide Nanomaterials
Manganese oxides are of immense industrial importance in a variety of new and old applications. Our interest in these compounds began with an initial synthesis from crystalline starting materials of lamellar manganese dioxides, which possessed a nanostructured microsphere morphology. We are currently investigating catalytic an capacitive properties of these materials.
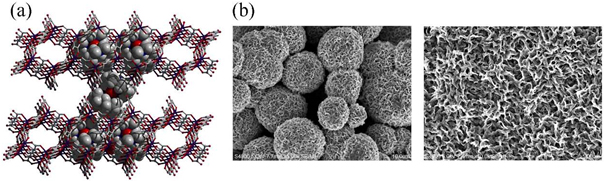
Related publications
- S. Acharya, B. Das, U. Thupakula, K. Ariga, D. D. Sarma, J. Israelachvili and Y. Golan. “A Bottom-up Approach Towards Fabrication of Ultrathin PbS Sheets” Nano Letters (2013) 13, 409–415. [Link
 ]
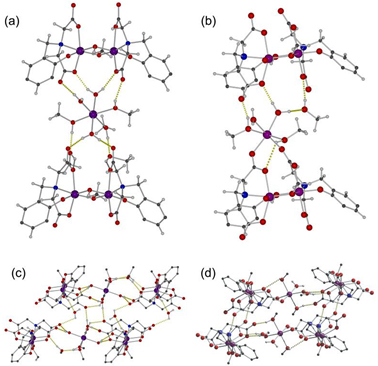
] - N. M. Sanchez-Ballester, L. K. Shrestha, M. R. J. Elsegood, K. Ariga, C. E. Anson, W. Schmitt, J. P. Hill and A. K. Powell. “Ligand Displacement for Fixing Manganese: Relevance for Cellular Metal Ion Transport and Synthesis of Polymeric Coordination Complexes” Dalton Trans. (2013) 42, 2779–2785. [Link
 ]
] - H. Palza, A. Maturana, F. Gracia, A. Neira, V. Fuenzalida, J. Avila, N. M. Sanchez-Ballester, M. R. J. Elsegood, S. J. Teat, J. P. Hill and K. Ariga. “Nanostructured Manganese Oxide Particles from Coordination Complex Decomposition and their Catalytic Properties for Ethanol Oxidation” J. Nanosci. Nanotechol. (2012) 12, 8087–8093. [Link
 ]
] - N. Pradhan, S. Acharya, K. Ariga, N. S. Karan, D. D. Sarma, Y. Wada, S. Efrima and Y. Golan. “Chemically Programmed Ultrahigh Density Two-Dimensional Semiconductor Superlattice Array” J. Am. Chem. Soc., (2010), 132, 1212–1213. [Link
 ]
] - S. Mandal, M. Sathish, G. Saravanan, K. K. R. Datta, Q. Ji, J. P. Hill, H. Abe, I. Honma and K. Ariga. “Open-Mouthed Metallic Microcapsules: Exploring Performance Improvements at Agglomeration-Free Interiors” J. Am. Chem. Soc. (2010) 132, 14415–14417. [Link
 ]
] - S. Kundu, J. P. Hill, G. J. Richards, K. Ariga, A. H. Khan, U. Thupakula and S.
Acharya. “Ultra-narrow PbS Nanorod-Nematic Liquid Crystal Blend for Enhanced Electro-optic Properties” ACS Appl. Mater. Interfaces (2010), 2, 2759–2766. [Link ]
] - S. Acharya, S. Kundu, J. P. Hill, G. J. Richards, K. Ariga. “Nanorod Driven Orientational Control of Liquid Crystal for Polarization-Tailored Electro-optic Devices” Adv. Mater. (2009), 21, 989–993. [Link
 ]
] - J. P. Hill, S. Alam, K. Ariga, C. E. Anson and A. K. Powell. “Nanostructured Microspheres of MnO2 by Room Temperature Solution Processing” Chem. Commun. (2008) 383–385. [Link
 ]
] - J. P. Hill, H. Palza, S. Alam, K. Ariga, A. L. Schumacher, F. D’Souza, C. E. Anson and A. K. Powell. “Decomposition of Dinuclear Manganese Complexes for Preparation of Nanostructured Oxide Materials” Inorg. Chem. (2008) 18, 8306–8314. [Link
 ]
] - I. McKeogh, J. P. Hill, E. S. Collins, T. McCabe, A. K. Powell and W. Schmitt. “Self-Assembly of Fe(III) Complexes via Hydrogen Bonded Water Molecules into Supramolecular Coordination Networks” New. J. Chem. (2007) 31, 1882–1886. [Link
 ]
] - W. Schmitt, J. P. Hill, S. Malik, C. A. Volkert, I. Ichinose, C.E. Anson and A. K. Powell. “Thermolysis of a Hybrid Organic-Inorganic Supramolecular Coordination Assembly: Templating the Formation of Nanostructured Fibrous Materials and C-based Microcapsules” Angew. Chem., Int. Ed. (2005) 44, 7048–7053. [Link
 ]
] - W. Schmitt, J. P. Hill, P. Juanico, A. Caneschi, F. Costantino, C. E. Anson and A. K. Powell.
“Supramolecular Coordination Assemblies of Dinuclear FeIII Complexes” Angew. Chem., Int. Ed. (2005) 44, 4187–4192. [Link ]
]