RESEARCH
1. Interfacial Science of Molecular Films
Molecules can be assembled with minute precision at interfaces as can be seen in self-assembled monolayers at metal surfaces or in highly ordered Langmuir-Blodgett and Langmuir-Schaeffer films.
In the case of metal surfaces, we have investigated the effect of molecular structure on packing modes and molecular mobility. Currently, we are focusing on properties of single molecules, either isolated or contained within a film, for use as molecular memory or switching devices.
On the other hand, molecules contained within Langmuir-Blodgett films have the unique feature that they may be manipulated by applying mechanical force (through in situ compression and expansion of the monolayer). Thus, molecular-machine-like functions can be obtained at the nanometric (or smaller!) level through actions performed at the macroscale leading us to coin the term ‘Hand-operated Nanotechnology’.
Molecular Machines at Interfaces
By employing an air water interface it is possible to demonstrate molecular level action through application of a macroscale force, in this case for the capture and release of fluorescent guest molecules. As is shown below, macroscale manipulation of a molecular monolayer at an air-water interface can be used to capture guest molecules in the water subphase since the molecular conformation is coupled with monolayer compression (and expansion – i.e. it is a reversible process).
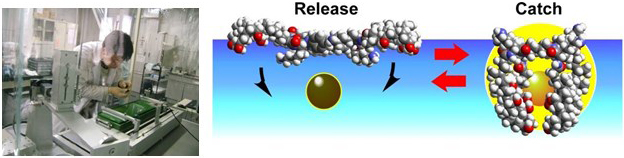
Chiral Resolution by Applying Macroscale Force
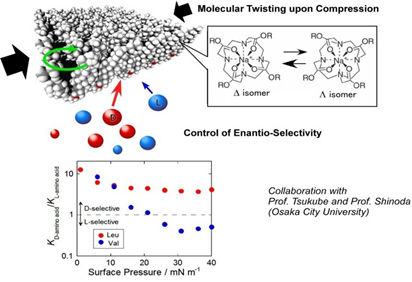
enantioselectivity of guest binding depending on the applied surface pressure.
Chirality of amino acids can be differentiated using force applied to monolayer films of a cholesterol-substituted cyclen derivative. By varying the pressure applied to this film, enantioselectivity of guest binding was observed.
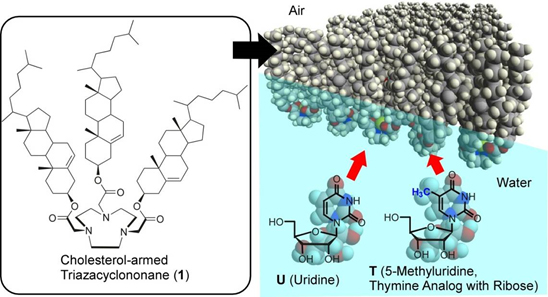
Nucleoside Differentiation using a Molecular Machine
Subtle structural differences of nucleoside guests can be detected using a cholesterol-substituted triazacyclononane molecular machine operated at an air-water interface. Thus, the single methyl group difference between uridine and 5-methyluridine can be found by mechanical tuning due to monolayer compression.
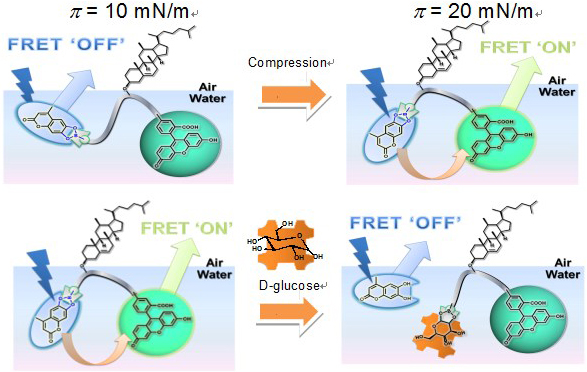
Mechanically-controlled Indicator Displacement Assay
Indicator displacement assay has been applied in a Langmuir-Blodgett film to detect carbohydrates using fluorescence resonance energy transfer (FRET).
Related Publications
- K. Sakakibara, L. A. Joyce, T. Mori, T. Fujisawa, S. H. Shabbir, J. P. Hill, E. V. Anslyn and K. Ariga. “Mechanically-Controlled Indicator Displacement Assay (MC-IDA)” Angew.Chem. Int. Ed. (2012) 51, 9643–9646. [Link
 ]
] - K. Ariga, T. Mori and J. P. Hill. “Evolution of Molecular Machines: from Solution to Soft Matter Interface” Soft Matter (2012), 8, 15–20. [Link
 ]
] - T. Michinobu, S. Shinoda, T. Nakanishi, J. P. Hill, K. Fujii, T. N. Player, H. Tsukube and K. Ariga. “Langmuir Monolayer of Cholesterol-Armed Cyclen Complex That Can Control Enantioselectivity of Amino Acid Recognition by Surface Pressure”Phys. Chem. Chem. Phys. (2011) 13, 4895–4900. [Link
 ]
] - K. Ariga, S. Ishihara, H. Izawa, H. Xia and J. P. Hill. “Operation of Micro- and Molecular machines: A New Concept with its Origins in Interface Science”Phys. Chem. Chem. Phys. (2011) 13, 4802–4811. [Link
 ]
] - T. Mori, K. Okamoto, H. Endo, K. Sakakibara, J. P. Hill, S. Shinoda, M. Matsukura, H. Tsukube, Y. Suzuki, Y. Kanekiyo and K. Ariga. “Mechanical Tuning of Molecular Machines for Nucleotide Recognition at the Air-Water Interface” Nanoscale Res. Lett.(2011) 6, 304. [Link
 ]
] - T. Mori, K. Okamoto, H. Endo, J. P. Hill, S. Shinoda, M. Matsukura, H. Tsukube, Y. Suzuki, Y. Kanekiyo and K. Ariga. “Mechanical Tuning of Molecular Recognition to Discriminate the Single-Methyl-Group Difference between Thymine and Uracil” J. Am. Chem. Soc. (2010) 132, 12868–12870. [Link
 ]
] - T. Michinobu, S. Shinoda, T. Nakanishi, J. P. Hill, K. Fujii, T. N. Player, H. Tsukube and K. Ariga. “Mechanical Control of Enantioselectivity of Amino Acid Recognition by a Cholesterol-Armed Cyclen Monolayer at the Air-Water Interface” J. Am. Chem. Soc. (2006) 128, 14478–14479. [Link
 ]
] - K. Ariga, T. Nakanishi, J. P. Hill, Y. Terasaka, D. Sakai and J.-I. Kikuchi. “Effect of Guest Capture Modes on Molecular Recognition by a Dynamic Cavity Array at the Air-Water Interface: Soft vs. Tight and Fast vs. Slow” Soft Matter (2005) 1, 132–137. [Link
 ]
] - K. Ariga, T. Nakanishi and J. P. Hill. “A Paradigm Shift in the Field of Molecular Recognition at the Air-Water Interface: From Static to Dynamic” Soft Matter (2006) 2, 465–477. [Review] [Link
 ]
]