Research
At Frontier Molecules Group, we are conducting research aimed at creating novel molecules with "advanced molecular functions" and using these molecules as major components for various applications. Room-temperature liquid π-conjugated molecules and π-conjugated polymers that can tune luminescent properties, π-liquids and π-gels that can stably maintain electrostatic charges (PI: Nakanishi), supramolecular polymers and organic/inorganic hybrid materials that enable gas detection (PI: Ishihara), π-conjugated macromolecules that dynamically change structure and function in response to mechanical stimuli, light, and heat (PI: Nagura), crystalline low-dimensional organic materials for water purification membranes and expressing unique electronic properties (PI: Matsumoto), and surface/interface design and droplet engineering related to droplet wetting behavior control (PI: Tenjimbayashi) are being carried out in our group. In addition, we conduct research using various techniques such as organic synthesis, spectrometry, self-assembly/disassembly, supramolecular chemistry, liquid chemistry, nano surface and interface chemistry, sensors, separation-purification, and coatings as essential chemical technologies. Below, we will introduce each group member's research on the excellent functionality of organic materials and the development of highly original molecular materials.
Functional Molecular Liquids
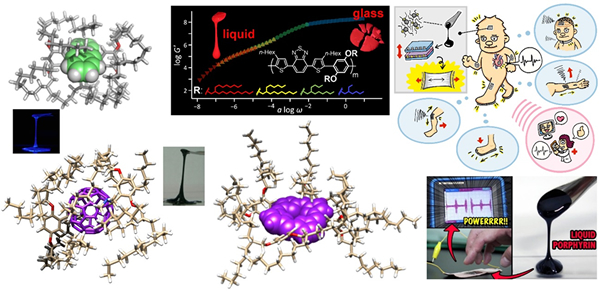
If the unique electronic properties of π-conjugated molecules can be reflected in bulk molecular materials, the potential for material applications of existing and newly developed π-conjugated molecules will be significantly expanded. Dr. Nakanishi is engaged in research on the "room-temperature liquefaction" of π-conjugated molecules and polymers and the creation of functional bulk organic materials. The room-temperature liquefaction strategy involves covalently bonding a bulky yet flexible branched long-alkyl chains onto the π skeleton and liquefying the π-conjugated molecule without a solvent using the ultra-high entropy derived from the side chains. By isolating adjacent π-conjugated molecules, bulk organic liquid materials can be created with optoelectronic properties unique to π-conjugations. So far, we have succeeded in turning various π-conjugated molecules and conjugated polymers, such as fullerene, anthracene, pyrene, naphthalene, porphyrin, and phthalocyanine, into room-temperature liquids and even gels (using the π liquid as the solvent). These alkyl-π liquids and alkyl-π gels are attracting attention as a new generation of functional soft materials that exhibit optoelectronic functions such as luminescence, photoconductivity, electrochromic, and electret properties.
I will explain “electrets” in a special feature. Charging a branched alkylated π molecular liquid makes it possible to create a π-liquid electret that stably retains electrostatic charge. Using this π-liquid (π-gels are also possible!) electret as a main component, a stretchable and freely deformable vibration power generator can be constructed. We are currently researching the development of a wearable weak vibration sensor that will contribute to healthcare applications.
[PI: Prof. Dr. Takashi Nakanishi]
[References]
J. Am. Chem. Soc. 2006, 128, 10384–10385. [link]
Nat. Commun. 2013, 4, 1969. [link]
Nat. Chem. 2014, 6, 690. [link]
Sci. Rep. 2017, 7, 3416. [link]
Chem. Sci. 2018, 9, 6774–6778. [link]
Angew. Chem. Int. Ed. 2019, 58, 9581–9585. [link]
Nat. Commun. 2019, 10, 4210. [link]
Mater. Horiz. 2023, 10, 3458–3466. [link]
Angew. Chem. Int. Ed. 2024, 63, e202402874. [link]
Gas management materials
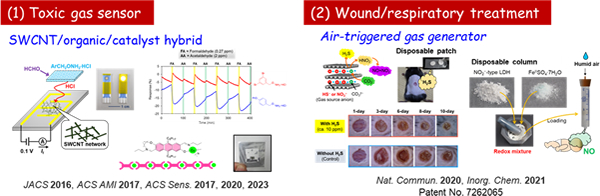
Despite simple structures of gas molecules, they are ubiquitous and deeply associated with various social issues (e.g., energy, climate change, environmental safety, medical care, and food). In this project, we are developing nanomaterials and devices toward precise management of gas molecules (e.g., sensing, controlled-release, and conversion). For example, (1) small sensors capable of detecting toxic gases have been developed based on the hybridization of single-walled carbon nanotube (SWCNT) and organic/catalytic selectors. Also, (2) solid materials that steadily release biologically-active gases (H2S and NO) have been developed by utilizing specific anion exchange properties of layered inorganic materials.
[PI: Dr. Shinsuke Ishihara]
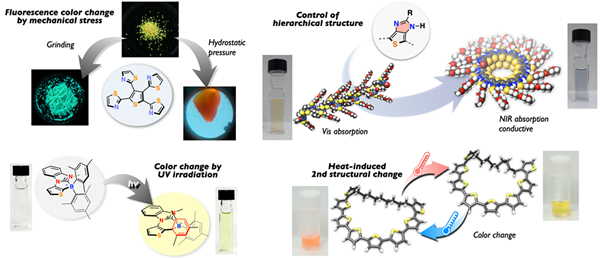
Development of Hierarchically Controlled π-Conjugated Macromolecules
Hierarchical structure of π-conjugated macromolecules makes significant impacts on their optical and electronic properties, as well as the performance of organic electronic devices. The developments of design principles and synthetic methodology, which enable the control of hierarchical structure precisely, are still challenging issues. In this work, we are developing novel organic macromolecules exhibiting unique π-electron functions (absorption, emission, conductivity, and stimuli responsiveness) by designing molecular structures hieratically.
[PI: Dr.Kazuhiko Nagura]
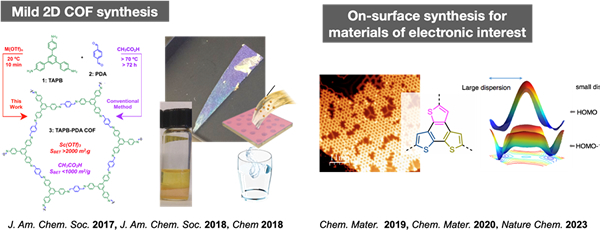
Crystalline low-dimensional materials
All materials consist of numerous atoms. However, the precise arrangement of those atoms in a material space with appropriate control remains a grand challenge even with the most advanced techniques of chemistry. In this research, we are trying to overcome this challenge by using covalent organic frameworks and molecular crystallization techniques to synthesize materials with novel structures in which the atomic arrangement is precisely determined in one or two dimensions, and to explore their functions. For instance, we are (1) developing a mild synthesis method for two-dimensional polymers and using the resulting membranes for water purification, and (2) developing electronic properties of network polymers that can be fabricated on metal surfaces.
[PI: Dr. Michio Matsumoto]
[References]
J. Am. Chem. Soc. 2017, 139, 4999–5002. [link]
J. Am. Chem. Soc. 2018, 140, 12677–12681. [link]
Chem 2018, 4, 308–317. [link]
Chem. Mater. 2019, 31, 3051–3065. [link]
Chem. Mater. 2020, 32, 10688–10696. [link]
Nat. Chem. 2023, 15, 136–142. [link]
J. Polym. Sci. 2023, 61, 861–869. [link]
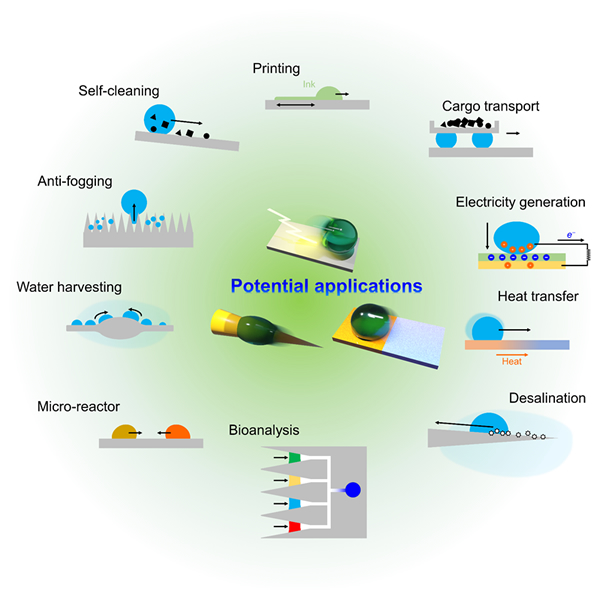
Modulating wettability of droplets
The wetting behavior of droplets relates with water repellency, water transport, industrial processes such as inkjet printing and environmental problems such as condensation, freezing and pollution. The wetting behavior widely depending on their physical properties, shape and formation process, the modulation of wetting will be a useful seed technology in all fields. Our aim is to design materials that can freely manipulate the interaction with the droplet interface, based on nanomaterial self-assembly technology. We mainly deal with droplet adhesion, coalescence/splitting, and formation dynamics. We have developed liquid-repellent coatings: anti-fouling, anti-ice, anti-rust, anti-bacterial, anti-fogging coatings, oil-water separation technology, droplet handling technology: microreactors, one-cell analysis tools, micro-templates, liquid structuring, liquid and pioneering new functional soft materials based on liquids.
[PI: Dr. Mizuki Tenjimbayashi]
[References]
Sci. Tech. Adv. Mater. 2022, 23, 473–497. [link]




