5. Chiral Sensing
Determining enantiomeric excesses of chiral acids (and now other compounds! Coming Soon!).
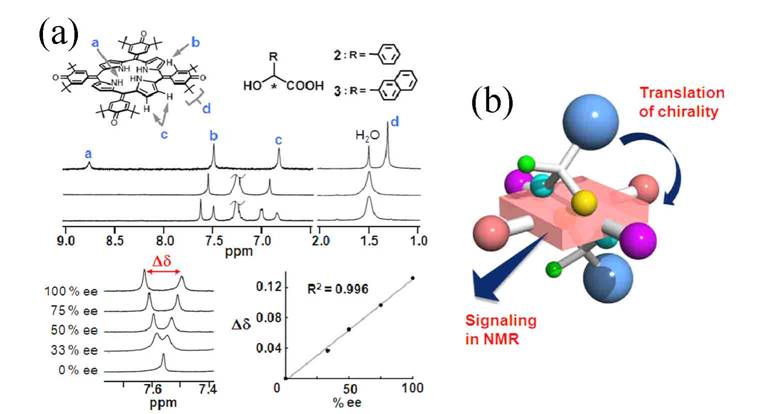
Figure 6. (a) Proton nuclear magnetic resonance analysis of enantiomeric excess using an achiral oxoporphyrinogen. (b) Artisitc rendition of the binding mechanism of chiral recognition.
Related publications.
1. Nuclear Magnetic Resonance Signaling of Molecular Chiral Information using an Achiral Reagent. A. Shundo, et al. J. Am. Chem. Soc. (2009) 131, 9494-9495.
2. Mechanical Control of Enantioselectivity of Amino Acid Recognition by a Cholesterol-Armed Cyclen Monolayer at the Air-Water Interface. T. Michinobu, et al. J. Am. Chem. Soc. (2006) 128, 14478-14479.
3. Chiral Recognition at the Air-Water Interface. K. Ariga, et al. Curr. Opin. Col. Int. Sci. (2008) 13, 23-30.
4. NMR Sensing of Chirality by Non-chiral Porphines. J. Labuta et al.Chem. Eur. J. (2011) in press.