Joint Workshop LANL/NIMS Quantum and Functional Materials and MANA International Symposium 2024
Session 9-1
Abstract
In the last decade, considerable effort has been devoted to develop high performance and durable anion exchange membranes (AEMs) for energy device applications such as alkaline fuel cells and water electrolyzers, and redox flow batteries. When these devices are operated under the alkaline conditions, use of non-precious metal catalysts becomes practical. Although a number of AEMs have been proposed in the literature, chemical and physical stability in alkaline media still remains an issue for long-term, reliable operation of the devices. In the pursuit of more stable, more ion-conductive AEMs, we have developed a series of new polymer structures. Combination of partially fluorinated polymer main chain free from heteroatom linkages such as ether, sulfide, and sulfone, and pendent ammonium head groups contributed to improvement of the stability as well as the ion conductivity of AEMs.
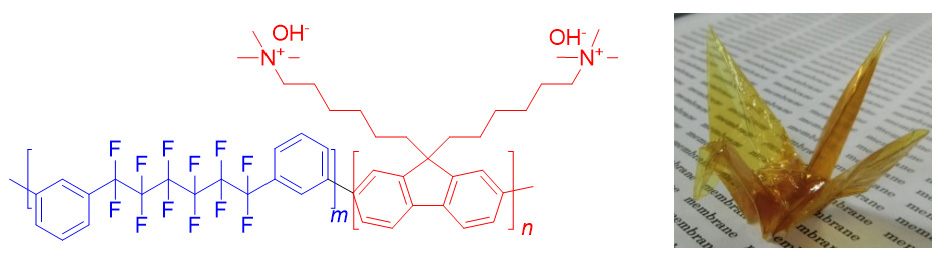
Reference
- H. Ono et al., J. Mater. Chem. A, 5, 24804 (2017). DOI: 10.1039/C7TA09409D
- E. J. Park et al., Chem. Soc. Rev., 53, 5704 (2024). DOI: 10.1039/D3CS00186E
- G. Shi et al., ACS Catal., 12, 14209 (2022). DOI: 10.1021/acscatal.2c02586
- V. Yadav et al., J. Mater. Chem. A, 12, in press (2024).

