Joint Workshop LANL/NIMS Quantum and Functional Materials and MANA International Symposium 2024
Plenary Talk 2
Abstract
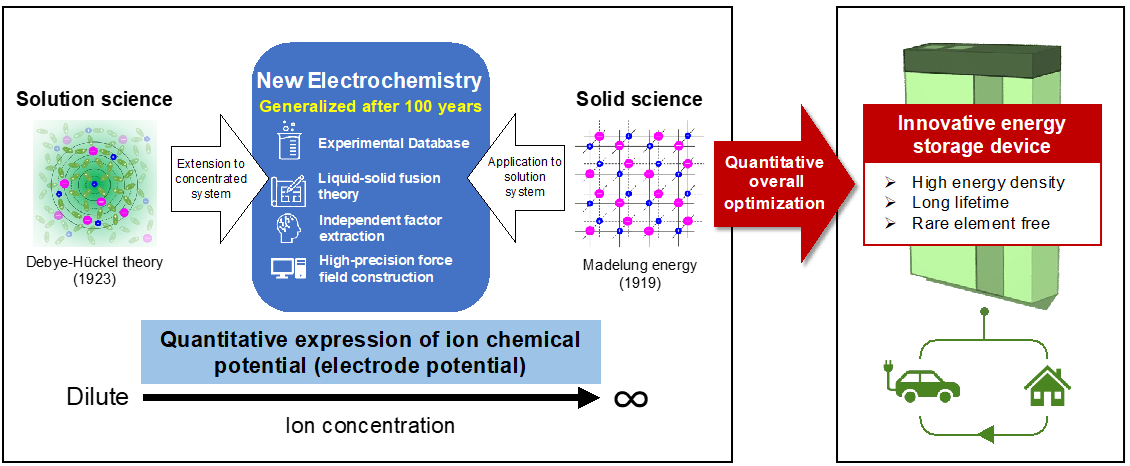
In any electrochemical system, electrode potential is the central variable that regulates the driving force of redox reactions. However, quantitative understanding of the electrolyte dependence has been limited to the classic Debye-Hückel theory that approximates the Coulombic interactions in the electrolyte under the dilute limit conditions. Therefore, accurate expression of electrode potential for practical electrochemical systems has been a holy grail of electrochemistry research for over a century since the introduction of the Debye-Hückel theory in 1923 that is valid only for electrolytes in the limit of infinite dilution. Tremendous efforts have been devoted to extend the Debye-Hückel theory to practical concentration region, however, explicit treatment of the long-range Coulombic interaction in highly fluctuating many-body liquid system has long been unrealistic, until the recent advances and maturity in molecular dynamics (MD) simulations. We have established a microscopic and rigorous description for the electrode potential that does not rely on phenomenological derivation applied thus far
Reference
- N. Takenaka, S. Ko, A. Kitada, A. Yamada, Nature Communications, 15, 1319 (2024). DOI: 10.1038/s41467-023-44582-4
- S. Ko, N. Takenaka, A. Kitada, A. Yamada, Next Energy, 1, 100014 (2023). DOI: 10.1016/j.nxener.2023.100014
- S. Ko, N. Takenaka, M. Nakayama, et al., A. Yamada, Nature Energy, 7, 1217 (2022). DOI: 10.1038/s41560-022-01144-0
- S. Ko, N. Takenaka, et al., A. Yamada, Nature Sustainability, 6, 1705 (2023). DOI: 10.1038/s41893-023-01237-y

