Joint Workshop LANL/NIMS Quantum and Functional Materials and MANA International Symposium 2024
Nanomaterials - 11
Abstract
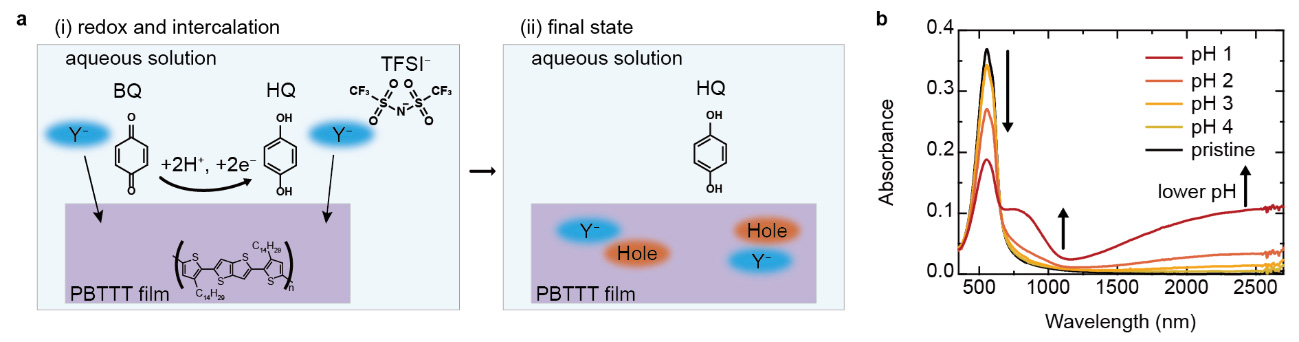
Chemical doping methods based on redox reactions for molecular semiconductors have been developed to enhance the properties of optoelectronic devices. However, maximizing the device performance requires precise control of the doping levels at the thermal energy scale (25 meV), which is challenging due to the difficulty in finely tuning the redox potential of the dopants. In addition, conventional dopants are prone to react with water or oxygen in air, which reduces controllability and reproducibility even in an inert atmosphere. These challenges present a significant bottleneck for the industrial fabrication of advanced OSC-based devices.
In this study, the doping levels of molecular semiconductors were precisely and reproducibly controlled in aqueous solutions under ambient conditions
Reference
- M. Ishii, Y. Yamashita, S. Watanabe, K. Ariga, J. Takeya, Nature 622, 285 (2023) DOI 10.1038/s41586-023-06504-8

