Joint Workshop LANL/NIMS Quantum and Functional Materials and MANA International Symposium 2024
Nanomaterials - 02
Abstract
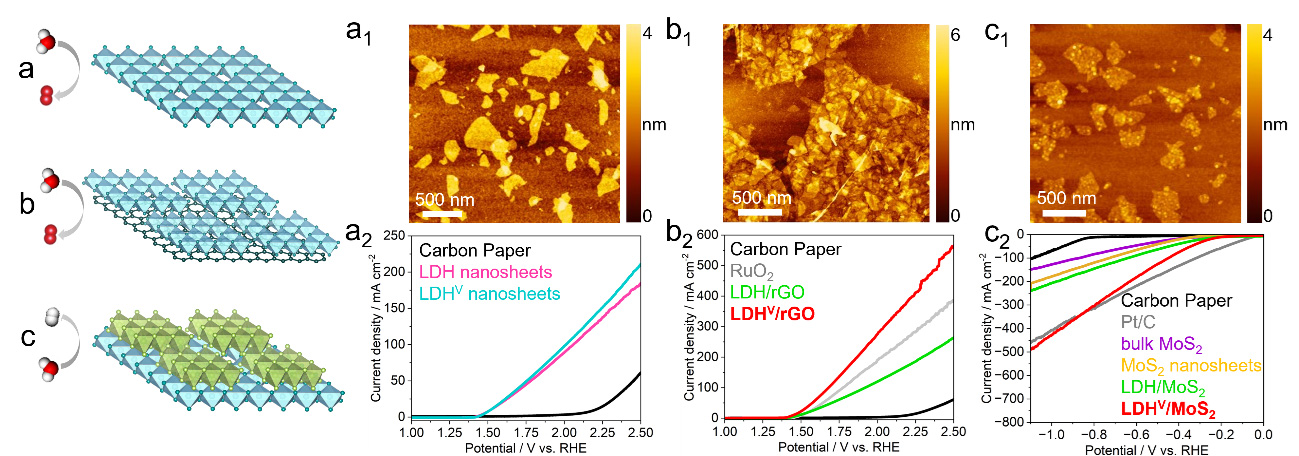
Hetero-assembly of different nanosheets and the interfacial synergistic effect are expected to enhance catalytic activity of the resultant composites.
Reference
- P. Xiong, R. Ma, T. Sasaki, et al., Nano Lett. 19, 4518−4526 (2019), DOI 10.1021/acs.nanolett.9b01329
- Q. Xie, Y. Kuang, X. Sun, et al., Nano Res. 11(9): 4524–4534 (2018), DOI 10.1007/s12274-018-2033-9

