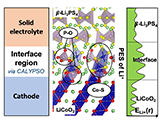
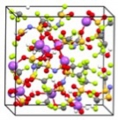
We used the CALYPSO method to investigated the mechanism of Li+ transport at the LiCoO2 cathode/β-Li3PS4 electrolyte interface. The results show that the interfacial reaction layer is formed with both mixing of cations and mixing of anions, and the interfacial Li+ sites with higher Li chemical potentials values cause the Li+ depletion in the initial stage of charging. This work provides a theoretical explanation of the interfacial resistance, and a perspective for the structure sampling of the heterogeneous solid/solid interfaces.
Bo Gao, Randy Jalem, Yoshitaka Tateyama* et al., Chem. Mater. 32, 85-96 (2020). "Li+ Transport Mechanism at the Heterogeneous Cathode/Solid Electrolyte Interface in an All-Solid-State Battery via the First-Principles Structure Prediction Scheme"
Selected research outputs
Solid electrolyte-electrode interface, Structure prediction, All-solid-state battery
Solid electrolyte-electrode interface, Interface stability, All-solid-state battery
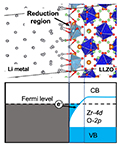
We investigated the chemical stability of the interface between Li7La2Zr2O12 (LLZO) and Li metal. We found that the coordinatively unsaturated Zr sites exist widely on the LLZO surfaces, and can be reduced once the LLZO surface is in contact with the Li metal, leading to chemical instability of the LLZO/Li interface. The present analysis provides a novel perspective for the rational optimization of the promising LLZO/Li interface.
Bo Gao, Randy Jalem, Yoshitaka Tateyama*, ACS Appl. Mater. Interfaces 12, 16350-16358 (2020). “Surface-Dependent Stability of the Interface between Garnet Li7La3Zr2O12 and the Li Metal in the All-Solid-State Battery from First-Principles Calculations”
Bo Gao, Randy Jalem, Yoshitaka Tateyama*, ACS Appl. Mater. Interfaces 12, 16350-16358 (2020). “Surface-Dependent Stability of the Interface between Garnet Li7La3Zr2O12 and the Li Metal in the All-Solid-State Battery from First-Principles Calculations”
Efficient material search, solid electrolyte, DFT, Bayesian optimization
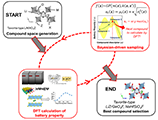
Materials search by DFT methods offers great promise for finding new solid electrolytes but the evaluation is known to be computationally expensive, particularly on ion migration property. A Bayesian-optimization-driven DFT-based approach was developed to efficiently screen for compounds with low ion migration energies (Eb), the latter being calculated by nudged elastic band (NEB) method. The validation of the proposed approach was demonstrated on 318 tavorite-type Li- and Na-containing compounds. We found that the scheme only requires ~30% of the total DFT-Eb evaluations on the average to recover the optimal compound ~90% of the time. Its recovery performance for desired compounds in the tavorite search space is 2× more than random search (i.e., for Eb < 0.3 eV).
Randy Jalem, Kenta Kanamori, Ichiro Takeuchi, Masanobu Nakayama, Hisatsugu Yamasaki, Toshiya Saito, Sci. Rep. 8, 5845 (2018). "Bayesian-Driven First-Principles Calculations for Accelerating Exploration of Fast Ion Conductors for Rechargeable Battery Application"
Randy Jalem, Kenta Kanamori, Ichiro Takeuchi, Masanobu Nakayama, Hisatsugu Yamasaki, Toshiya Saito, Sci. Rep. 8, 5845 (2018). "Bayesian-Driven First-Principles Calculations for Accelerating Exploration of Fast Ion Conductors for Rechargeable Battery Application"
Crystalline solid representation, descriptors, machine learning, battery materials
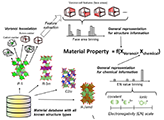
A general material representation that can effectively encode characteristics of inorganic materials or uniquely differentiate among them is needed for an effective large-scale screening of battery materials based on various target properties. A histogram-based representation for crystalline solids, which is invariant to atom ordering, cell periodicity, and number of atoms, was developed by binning and Voronoi-tessellation real-value features and atomic/chemical property data. This approach has resulted to a better predictive power and generalization performance of machine models relative to RDF-based descriptor vector on a thousand-scale dataset of DFT-calculated properties such as cohesive energy, density, band gap, and decomposition energy. From variable importance analysis, it was confirmed that binned atomic/chemical property and 2NN Voronoi cell geometric property data are good predictors.
Randy Jalem, Masanobu Nakayama, Yusuke Noda, Tam Le, Ichiro Takeuchi, Yoshitaka Tateyama, Hisatsugu Yamasaki, Sci. Tech. Adv. Mater. 19, 231-242 (2018). "A General Representation Scheme for Crystalline Solids based on Voronoi-Tessellation Real Feature Values and Atomic Property Data"
Randy Jalem, Masanobu Nakayama, Yusuke Noda, Tam Le, Ichiro Takeuchi, Yoshitaka Tateyama, Hisatsugu Yamasaki, Sci. Tech. Adv. Mater. 19, 231-242 (2018). "A General Representation Scheme for Crystalline Solids based on Voronoi-Tessellation Real Feature Values and Atomic Property Data"
Kinetics, Catalyst
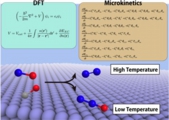
Atsuhi Ishikawa* , Yoshitaka Tateyama*, J. Phys. Chem. C 122, 17378-17388 (2018). "First-Principles Microkinetic Analysis of NO + CO Reactions on Rh(111) Surface toward Understanding NOx Reduction Pathways"
Na-excess cathode, O redox, Battery
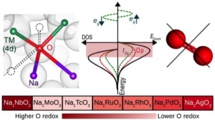
M. H. N. Assadi, Yoshitaka Tateyama* et al., J. Mater. Chem. A 6, 3747-3753 (2018). “Oxygen redox in hexagonal layered NaxTMO3 (TM =4d elements) for high capacity Na ion batteries”
Superconcentrated electrolyte, Battery
Together with Prof. Yamada group in The Univ. of Tokyo, we have investigated microscopic characters and mechanisms of super concentrated electrolytes such as LiTFSA/AN, Hydrate melt, fire-extinguisher electrolyte etc., and been providing novel aspects regarding the battery electrolytes.
Wang Jianhui, Keitaro Sodeyama, Yoshitaka Tateyama, Atsuo Yamada et al., Nat. Energy 3, 22-29 (2018). “Fire-extinguishing organic electrolytes for safe batteries”: Yuki Yamada, Keitaro Sodeyama, Yoshitaka Tateyama, Atsuo Yamada et al., Nat. Energy 1, 16129 (2016). “Hydrate-melt electrolytes for high-energy-density aqueous batteries”: Jianhui Wang, Yuki Yamada, Keitaro Sodeyama, Yoshitaka Tateyama, Atsuo Yamada et al., Nat. Commun. 7, 12032 (2016). “Superconcentrated electrolytes for a high-voltage lithium-ion battery”
Wang Jianhui, Keitaro Sodeyama, Yoshitaka Tateyama, Atsuo Yamada et al., Nat. Energy 3, 22-29 (2018). “Fire-extinguishing organic electrolytes for safe batteries”: Yuki Yamada, Keitaro Sodeyama, Yoshitaka Tateyama, Atsuo Yamada et al., Nat. Energy 1, 16129 (2016). “Hydrate-melt electrolytes for high-energy-density aqueous batteries”: Jianhui Wang, Yuki Yamada, Keitaro Sodeyama, Yoshitaka Tateyama, Atsuo Yamada et al., Nat. Commun. 7, 12032 (2016). “Superconcentrated electrolytes for a high-voltage lithium-ion battery”
Ion migration (cation & anion), Degradation, Perovskite solar cell
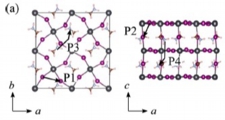
Jun Haruyama, Yoshitaka Tateyama* et al., J. Am. Chem. Soc. 137, 10048-10051 (2015). “First-Principles Study of Ion Diffusion in Perovskite Solar Cell Sensitizers”
Interface electron/hole transfer, Perovskite solar cell
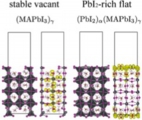
Jun Haruyama*, Yoshitaka Tateyama* et al., J. Phys. Chem. Lett. 8, 5840-5847 (2017). “First-Principles Study of Electron Injection and Defects at the TiO2/ CH3NH3PbI3 Interface of Perovskite Solar Cells”: Jun Haruayama, Yoshitaka Tateyama* et al., Acc. Chem. Res. 49, 554-561 (2016). “Surface Properties of CH3NH3PbI3 for Perovskite Solar Cells” : Jun Haruyama, Yoshitaka Tateyama* et al., J. Phys. Chem. Lett. 5, 2903-2909 (2014). “Termination Dependence of Tetragonal CH3NH3PbI3 Surfaces for Perovskite Solar cells”
Solid electrolyte - electrode interface, Ion depletion growth, Solid state battery
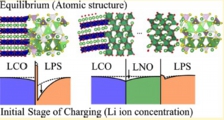
Jun Haruyama, Yoshitaka Tateyama* et al., ACS Appl. Mater. Interfaces 9, 286-292 (2017). “Cation Mixing Properties toward Co Diffusion at the LiCoO2 Cathode/ Sulfide Electrolyte Interface in a Solid-State Battery”: “Space-Charge Layer Effect at Interface between Oxide cathode and Sulfide Electrolyte in All-Solid-State Lithium-Ion Battery”, Jun Haruyama, Yoshitaka Tateyama* et al., Chem. Mater. 26, 4248-4255 (2014).
CeO2/Pt/H2O interface, Interfacial proton transfer, Catalyst
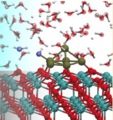
Boron-dope diamond electrode, Space-charge layer, Catalyst
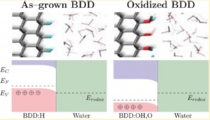
Takeshi Kashiwada, Yusuke Ootani, Yoshitaka Tateyama, Yasuaki Einaga et al., ACS Appl. Mater. Interfaces (2016). “A Study on Electrolytic Corrosion of Boron-Doped Diamond Electrodes when Decomposing Organic Compounds” : Zdenek Futera, Yoshitaka Tateyama* et al., J. Phys. Chem. C, 118, 22040-22052 (2014). "First Principles Calculation Study on Surfaces and Water Interfaces of Boron-Doped Diamond”
Reductive decomposition of electrolyte, SEI film formation & characteristics, Battery
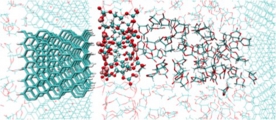
Yukihiro Okuno, Keisuke Ushirogata, Keitaro Sodeyama, Yoshitaka Tateyama*, Phys. Chem. Chem. Phys. 18, 8643-8653 (2016). “Decomposition of the fluoroethylene carbonate additive and the glue effect of lithium fluoride products for the solid electrolyte interphase: an ab initio study”: Keisuke Ushirogata, Keitaro Sodeyama, Yoshitaka Tateyama*, Yukihiro Okuno* et al., J. Electrochem. Soc. 162, A2670-A2678 (2015). “Near-Shore Aggregation Mechanism of Electrolyte Decomposition Products to Explain Solid Electrolyte Interphase Formation”: Keisuke Ushirogata, Keitaro Sodeyama, Yukihiro Okuno, Yoshitaka Tateyama*, J. Am. Chem. Soc. 135, 11967-11974 (2013). "Additive Effect on Reductive Decomposition and Binding of Carbonate-Based Solvent toward Solid Electrolyte Interphase Formation in Lithium-Ion Battery"
Theory, Methodology, Redox potential
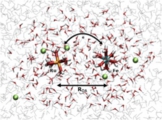
Zdenek Futera, Yoshitaka Tateyama* et al., Phys. Chem. Chem. Phys. 16, 19530-19539 (2014). “A double-QM/MM method for investigating donor-acceptor electron-transfer reactions in solution”: Ryota Jono, Yoshitaka Tateyama, Koichi Yamashita, Phys. Chem. Chem. Phys. 17, 27103-27108 (2015). “A method to calculate redox potentials relative to the normal hydrogen electrode in nonaqueous solution by using density functional theory-based molecular dynamics”: Ryota Jono, Yoshitaka Tateyama, Koichi Yamashita, J. Phys. Chem. Lett. 3, 3581-3584 (2012). “Redox Reaction Mechanisms with Non-triiodide Mediators in Dye-Sensitized Solar Cells by Redox Potential Calculations”
Superconcentrated electrolyte, Battery
“Sacrificial anion reduction mechanism for electrochemical stability improvement in highly concentrated Li-salt electrolyte”, Keitaro Sodeyama, Yuki Yamada, Koharu Aikawa, Atsuo Yamada, Yoshitaka Tateyama, J. Phys. Chem. C 118, 14091-14097 (2014).
"Unusual Stability of Acetonitrile-Based Superconcentrated Electrolytes for Fast-Charging Lithium-Ion Batteries", Y. Yamada, K. Furukawa, K. Sodeyama, K. Kikuchi, M. Yaegashi, Y. Tateyama, and A. Yamada, J. Am. Chem. Soc. 136 (2014):
Water configuration, Swelling
"Unusually Stable ~100-Fold Reversible and Instant Swelling of Inorganic Layered Materials", F. Geng, R. Ma, A. Nakamura, K. Akatsuka, Y. Ebina, Y. Yamauchi, N. Miyamoto, Y. Tateyama, and T. Sasaki, Nat. Commun. 4, 1632 (2013).
TiO2/water interface, TiO2/organic electrolyte interface, Catalyst, Solar cell
“Water contamination effect on liquid acetonitrile / TiO2 anatase (101) interface for durable dye-sensitized solar cell”, Masato Sumita, Keitaro Sodeyama, Liyuan Han, Yoshitaka Tateyama, J. Phys. Chem. C 115, 19849-19855 (2011).
Theory, Method, DFT-MD, Marcus theory, Thermodynamic integration, Redox potential
“Free energy calculation of water addition coupled to reduction of aqueous RuO4-”, Yoshitaka Tateyama, Jochen Blumberger, Takahisa Ohno, Michiel Sprik, J. Chem. Phys. 126, 204506 (2007):“Density-functional molecular-dynamics study of the redox reactions of two anionic, aqueous transition-metal complexes”, Yoshitaka Tateyama, Jochen Blumberger, Michiel Sprik, Ivano Tavernelli, J. Chem. Phys. 122, 234505 (2005).
Excited states dynamics, Method
“Real-time propagation time-dependent density functional theory study on the ring-opening transformation of the photoexcited crystalline benzene”, Yoshitaka Tateyama, Norihisa Oyama, Takahisa Ohno, Yoshiyuki Miyamoto, J. Chem. Phys. 124, 124507 (2006).
Hydrogen embrittlement, Hydrogen, Defect, Iron
“Stability and clusterization of hydrogen-vacancy complexes in alpha-Fe: An ab-initio study”, Yoshitaka Tateyama, Takahisa Ohno, Phys. Rev. B67, 174105 (2003): “Atomic-scale effects of hydrogen in iron toward hydrogen embrittlement: Ab-initio study”, Yoshitaka Tateyama, Takahisa Ohno, ISIJ Int. 43, 573 (2003)