Satellite Principal Investigator
Thomas E. Mallouk
The Mallouk group works at the interface of nanoscale materials and electrochemistry. Their research focuses on energy conversion and motion at the nanoscale.
New materials, surface chemistry, and bipolar membranes for energy conversion and storage in alkaline fuel cells and redox flow batteries
Current Topics
Bipolar membranes (BPMs) are being studied for applications in fuel cells, water and CO2 electrolysis, and pH swing devices for ocean capture of CO2. In redox flow batteries, a BPM can manage proton balance between acidic and basic electrolytes, effectively adding up to 800 mV to the cell voltage. Functionalizing graphitic electrodes by diazonium chemistry (Fig. 1) addresses the problem of their slow electron transfer kinetics in alkaline media. This strategy is being applied to carbon electrodes for various electrochemical energy conversion applications.
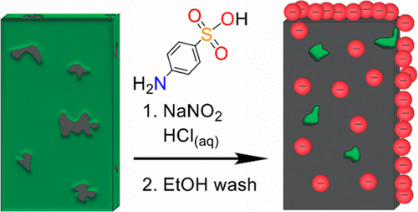
Fig. 1. Diazonium coupling can be used to functionalize carbon electrodes with hydrophilic groups (red).
Outline of Research
Alkaline electrochemical systems such as fuel cells, electrolyzers, redox flow batteries, and metal-air batteries offer an interesting alternative to acidic polymer membrane electrolyte (PEM) systems because they do not require precious metal electrocatalysts. We have studied layered double hydroxides as solid-state hydroxide ion (OH-) conductors for these devices and recently discovered that Ag2O also conducts OH- ions through the oxidative doping mechanism shown in Fig. 2. Ag2O is a promising alternative to polymeric ionomers in alkaline fuel cells because it is resistant to degradation by oxygen radical species.
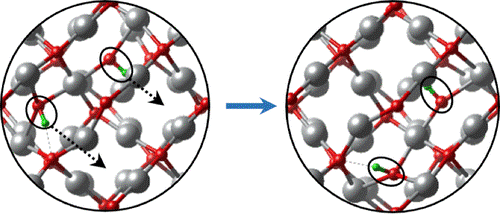
Fig. 2. Cooperative diffusion of pairs of OH- ions coordinated to Ag(III) sites in oxidatively doped Ag2O.
References
- L. Schulte et al., Chem. Mater. 36, 11440 (2024). DOI: 10.1021/acs.chemmater.4c02082
- A.S. Metlay et al., J. Am. Chem. Soc. 146, 20086 (2024). DOI: 10.1021/jacs.4c04088
Group members
-
 Thomas E. Mallouk・Principal Investigator
Thomas E. Mallouk・Principal Investigator


