MANA International Symposium 2025
Semiconductor Materials - 10
Abstract
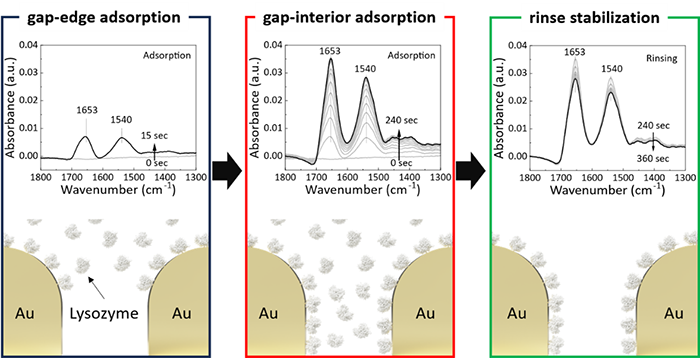
Understanding protein adsorption at solid-liquid interfaces is essential for advancing biosensing, biomaterials, and biocatalysis. In this study, we present a polarization-resolved attenuated total reflection surface-enhanced infrared absorption spectroscopy (ATR-SEIRA) platform utilizing gold near-percolated optimized structure. Using hen egg white lysozyme as a model, in situ ATR-SEIRA spectra with s-polarized light revealed three distinct adsorption stages—gap-edge adsorption, gap-interior adsorption, and rinse stabilization—each characterized by dynamic changes in the amide I/II intensity ratio. Post-rinse spectral deconvolution of the s-polarized amide I band revealed secondary structure changes: a 4.8% decrease in α-helix, a 9.7% reduction in random coil, and increases of 2.3% in β-sheet and 12.2% in β-turn content. These results highlight that the nanogap-enhanced SEIRA platform provides a highly sensitive and structurally informative tool to investigate protein adsorption and conformational dynamics under physiologically relevant conditions.