Gold Nanoparticle Catalyst that Learns from Enzyme in Nature
Metallic Nanoparticle Catalyst for Surface Capture of Reactants
2012.10.03
(2012.11.15 Update)
National Institute for Materials Science
A team led by Dr. Kazushi Miki, Group Leader of the Functional Heterointerface Group, Polymer Materials Unit, National Institute for Materials Science succeeded in development of a high activity gold nanoparticle catalyst that simplify the function of enzyme in capturing substances.
Abstract
- A team led by Dr. Kazushi Miki, Group Leader of the Functional Heterointerface Group, Polymer Materials Unit (Unit Director: Izumi Ichinose), National Institute for Materials Science (President: Sukekatsu Ushioda) succeeded in development of a high activity gold nanoparticle catalyst that simplify the function of enzyme in capturing substances.
- This new type of catalyst mimics enzyme, which supports biological activities as a catalyst in the reactions of the living body. Metalloenzymes has metal element which functions as a catalyst in the active center, and manifest extremely high activity and selectivity by possessing a function in which proteins surrounding the vicinity capture designated substances at activity sites. The NIMS group succeeded in realizing catalytic activity similar to that of metalloenzymes by simplifying the structure of these metalloenzymes in gold nanoparticles coated with alkanethiol molecules.
- In this work, the NIMS research group focused on the fact that a self-assembled alkanethiol monolayer formed on the surface of gold nanoparticles (AuNP) possesses an interaction similar to that of cell membranes (lipid bilayer), which capture molecules of designated lengths and shapes. Because the molecules which are captured on the particle surface by this interaction increase the probability of contact with the gold particle surface, which has a catalytic function, the catalytic reaction is accelerated. Concretely, a high activity catalytic reaction was discovered, in which silane molecules are efficiently activated on the surface of gold, which is a catalyst, by capture of silane molecules and alcohol molecules on the surface of the gold particles.
- As this result confirmed the mechanism of a catalytic reaction similar to that of metalloenzymes, it is expected to be possible to realize catalysts with a combination of high activity and high selectivity by designing modified molecules for AuNP. Furthermore, unlike natural enzyme, which can only be used stable in aqueous solutions, AuNP display extremely high chemical stability, enabling use under acidic and basic solution conditions and in organic solvents. Thus, there are no restrictions on industrial use.
- This research was carried out as part of the research subject “Spatial and Temporal Integration of Near Field Reinforced Photochemical Reactions” (FY 2010-2012; Research Representative: Kazushi Miki) in the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grants in Aid for Scientific Research on Innovative Areas research field “Organic Synthesis Based on Reaction Integration: Development of New Methods and Creation of New Substances” (Area Representative: Jun-ichi Yoshida, Professor, Kyoto University Graduate School of Engineering; http://www.sbchem.kyoto-u.ac.jp/syuuseki/index ). A patent application has already been filed in connection with this research. These results will soon be published in the journal of Advanced Materials (Wiley).
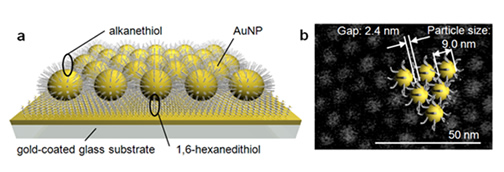
Fig : Schematic diagram of the new catalyst
(a) The new catalyst has a structure in which gold nanoparticles (AuNP) having a size of 10nm (1/100 millionth of 1m) coated with alkanethiol are regularly arranged on a flat substrate. (b) In this scanning electron microscope image, it can be understood that the actual size of the AuNP is 9.0nm, and the gap between the AuNP is 2.4nm.