Development of Drug Eluting Stent with Excellent Antithrombotic and Vascular Endothelium Formation Properties
National Institute for Materials Science
Graduate School of Medicine, The University of Tokyo
The Biomaterials Center of the NIMS, in joint work with the Graduate School of Medicine of The University of Tokyo and Nipro Corporation, succeeded in the development of a new drug eluting stent with excellent antithrombotic and vascular endothelium formation properties.
概要
- The Biomaterials Center (Managing Director: Yuji Miyahara) of the National Institute for Materials Science (hereinafter NIMS; President: Sukekatsu Ushioda), in joint work with the Graduate School of Medicine of The University of Tokyo (Professor: Ryozo Nagai) and Nipro Corporation (President: Minoru Sano), succeeded in the development of a new drug-eluting stent with excellent antithrombotic endothelialization properties.
- Drug-eluting stents (DES) are a key method of treating ischemic heart disease (IHD), as represented by angina pectoris and myocardial infarction. However, issues related to the polymer matrix for slow release of the drug and the drug itself remain to be solved. Thus, the development of a DES which possesses combined functions that promote endothelialization and antithrombogenicity and enables slow release of the drug with minimal side effects had been desired.
- NIMS and its partners in this research developed an Am80-loaded drug eluting stent by incorporating the drug Tamibarotene (Am80), which has a proven record of clinical use, in a citric acid-crosslinked alkali-treated gelatin polymer matrix, which displays antithrombogenic and endothelialization properties. A large amount of Am80 is slowly released during the first 1-2 weeks after expansion of the stent, when the inflammatory response is strong, and drug release continues for 8 weeks. When the prepared Am80-eluting stent was left for 2 weeks in a coronary artery of a pig, satisfactory endothelialization was observed, and absolutely no formation of clots (thrombus) was detected.
- The number of patients with ischemic heart diseases such as angina pectoris and myocardial infarction is increasing annually, accompanying the advent of an ultra-high age society. On the other hand, more than 90% of the DES used in Japan are manufactured by foreign companies. Because the DES developed by the NIMS-led team is based on unique Japanese basic technologies, it is expected to contribute to activation of the domestic medical device industry and the creation of new medical devices with international competitiveness.
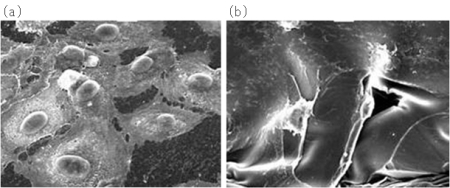
Fig. Vascular endothelium formation by polymer matrix
(a)Developed by NIMS (Endothelium formation)
(b)Commercialized product (No endothelium formation)