Research Achievements
1. Development of visible-light-responsive hydrogen-generating photocatalyst Sn3O4 that is abundantly and cheaply available
Sunlight is the ultimate form of sustainable energy, but it cannot yet replace conventional fossil and nuclear fuels, as no technology has been established for directly converting the sunlight into chemical energy (i.e., fuel), which is suitable for concentration and transportation. Many hydrogen-generating catalysts, e.g., titanium oxide (TiO2), are capable of generating hydrogen fuel from solutions of organic materials by absorbing ultraviolet rays. However, because visible light, which comprises the majority of solar light, cannot be absorbed, it is difficult to use for actual solar energy conversions. The development of new photocatalyst materials capable of decomposing water by absorbing visible light is progressing on a global scale, but there are issues regarding the cost and environmental responsiveness, as many of the current materials contain rare metals in high concentrations, such as tantalum, which is expensive, or lead, which is highly toxic.
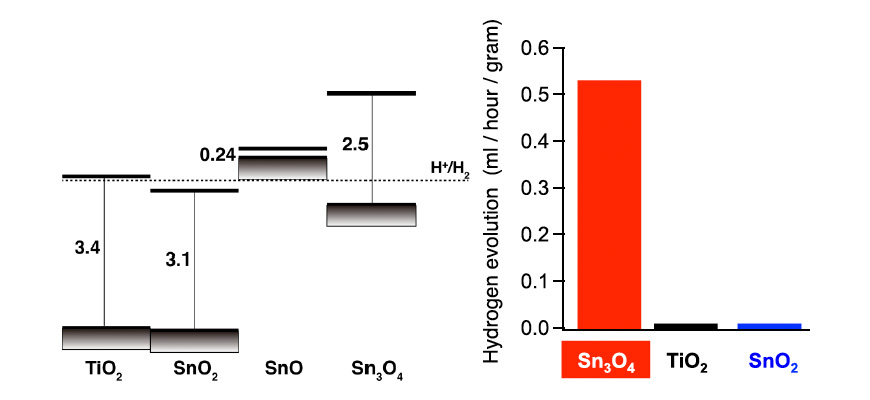
Fig. 1: Position of band edges of tin oxide (left) and hydrogen-generating photocatalytic activities of Sn3O4, TiO2, and SnO2 irradiated by visible light (λ > 400 nm) (right).
Our group conducted material developments according to theory, theoretically predicting that oxides that include the divalent tin ion (Sn2+) have electronic structures desirable for the hydrogen-generating reactions of photocatalysts irradiated by visible light. Proceeding with his material research in accordance with this guideline in collaboration with experimental researchers, we discovered a tin oxide comprising a divalent tin ion (Sn2+) and a tetravalent tin ion (Sn4+): Sn3O4 (Sn2+2 Sn4+O4) (ACS Applied Materials & Interfaces, 6, 3790-3793, 2014). The band alignment of TiO2, SnO2, SnO, and Sn3O4 derived by the first-principle calculation are shown on the left side of Fig. 1. It is evident that the position of the conduction band minimum for Sn3O4 is sufficiently higher than the reduction potential of water and is suitable for the hydrogen-generating reaction. This substance was revealed to generate hydrogen from solutions of organic materials irradiated by visible light, under which the TiO2 was not activated at all (right side of Fig. 1). Oxides of tin have a low toxicity, are inexpensive, and are available in abundance; therefore, they are widely used as materials for transparent conductors. Photocatalysts of Sn3O4 can reduce the environmental burden and manufacturing cost of hydrogen fuel and are expected to provide a significant contribution toward the realization of a recycling society based on solar energy.
Publications
— Maidhily Manikandan, Toyokazu Tanabe, Peng Li, Shigenori Ueda, Gubbala V. Ramesh, Rajesh Kodiyath, Junjie Wang, Toru Hara, Arivuoli Dakshanamoorthy, Shinsuke Ishihara, Katsuhiko Ariga, Jinhua Ye, Naoto Umezawa*, and Hideki Abe*,
"Photocatalytic Water Splitting under Visible Light by Mixed-Valence Sn3O4," ACS Applied Materials & Interfaces, 6, 3790-3793, 2014.
2. Theoretical design of a highly active SrTiO3-based photocatalyst from doping scheme toward solar energy utilization for hydrogen production
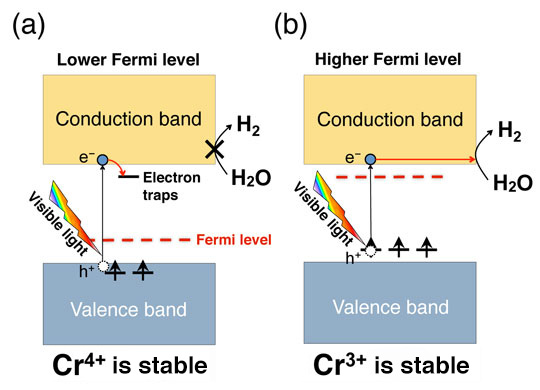
Fig. 2: Effect of Fermi level tuning on oxidation number of Cr and photocatalytic activity. (a) When the Fermi level is low, activity is reduced because unoccupied states caused by tetravalent Cr capture photoexcited electrons. (b) When the Fermi level is high, the water-splitting reaction is accelerated because the trivalent Cr becomes dominant and electron capture is inhibited.
Strontium titanate (SrTiO3) is expected to serve as a photocatalyst that enables the production of hydrogen solely using sunlight, owing to its high stability under light irradiation and its strong photoreduction ability. Nevertheless, because its optical absorption edge is located in the ultraviolet region, it cannot effectively use visible light, which constitutes the greatest part of sunlight. Thus, research has been conducted to adjust the absorption edge into the visible light region by doping the SrTiO3 with transition metals such as chromium (Cr). In recent years, considerable research has been performed on the co-doping of transition-metal elements and other elements with the aim of further enhancing the photocatalytic activity.
Cr-doped SrTiO3 has been known to exhibit a high hydrogen-generation efficiency when Cr is trivalent (Cr3+). This is because when Cr is tetravalent (Cr4+), the photocatalytic reaction is inhibited owing to the electron capture (Fig. 2). Therefore, in this research, we attempted to stabilize the Cr3+ by increasing the Fermi level by doping the Cr with various other elements. Using the electronic structure calculation based on the density-functional theory, we estimated the position of the Fermi level for the case where the Sr in the SrTiO3 is replaced with La and Y, Ti is replaced with Ta, Sb, and Nb, and O is replaced with F, on the basis of the charge neutrality condition. We also determined which dopant enhances the photocatalytic activity the most when co-doped with Cr. Thus, we predicted a substantial rise in the Fermi level and a high Cr3+ concentration when La, which has the capacity to form conduction electrons in SrTiO3, is co-doped with Cr (Fig. 3a). Our experiments confirmed that SrTiO3 co-doped with La and Cr exhibits a higher hydrogen-generation efficiency under visible-light irradiation than that co-doped with other elements, thereby demonstrating the validity of the theoretical prediction (Fig. 3b).
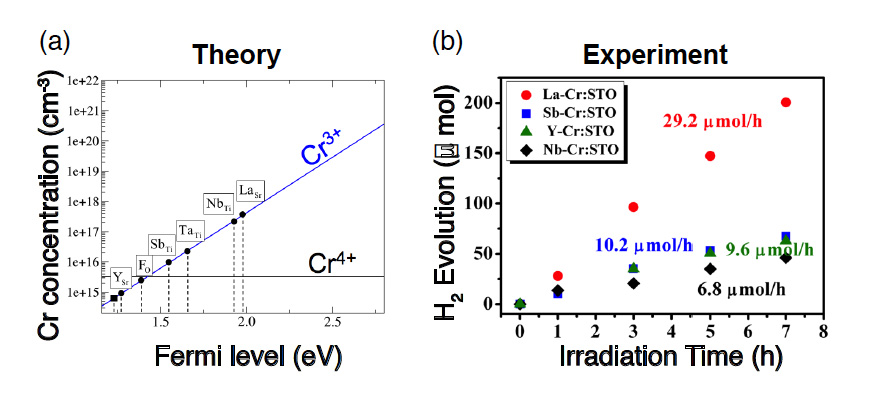
Fig. 3: Theoretical prediction of hydrogen-generation efficiency and verification experiment. (a) Relationship between position of the Fermi level and Cr3+ concentration after doping the respective elements. The Cr3+ concentration, which accelerates photocatalytic activity, was theoretically predicted to be the highest when Sr was replaced with La. (b) Experiment of hydrogen generation through water splitting under visible-light irradiation, using samples for which Cr and the respective elements (La, Sb, Y, or Nb) were co-doped into SrTiO3. It was experimentally confirmed that the activity is the highest when La and Cr are co-doped, thus demonstrating the validity of the theory.
Publications
— P. Reunchan, S. Ouyang, N. Umezawa, H. Xu, Y. Zhang, and J. Ye,
"Theoretical design of highly active SrTiO3-based photocatalysts by a codoping scheme towards solar energy utilization for hydrogen production," Journal of Materials Chemistry A, 1, 4221-4227, 2013.
— P. Reunchan, N. Umezawa, S. Ouyang, and J. Ye,
"Mechanism of photocatalytic activities in Cr-doped SrTiO3 under visible-light irradiation: an insight from hybrid density-functional calculations," Phys. Chem. Chem. Phys., 14, 1876-1880, 2012.
Presentations
— Naoto Umezawa, Pakpoom Reunchan, Shuxin Ouyang, Xu Hua, Yuanjian Zhang, and Jinhua Ye,
"Theoretical Design of Highly Active SrTiO3-based Photocatalyst from Doping Scheme toward Solar Energy Utilization for Hydrogen Production," 10th Pacific Rim Conference on Ceramic and Glass Technology, The American Ceramic Society, June 2013, San Diego, CA, USA (invited).
— Naoto Umezawa, Pakpoom Reunchan, Shuxin Ouyang, Xu Hua, Yuanjian Zhang, and Jinhua Ye,
"Theoretical design of highly active SrTiO3-based photocatalysts by a codoping scheme towards solar energy utilization for hydrogen production," The 8th Siam Physics Congress, Thai Physical Society, March 2013, Chiangmai, Thailand (invited).
3. Theoretical study of highly active photocatalyst Ag3PO4
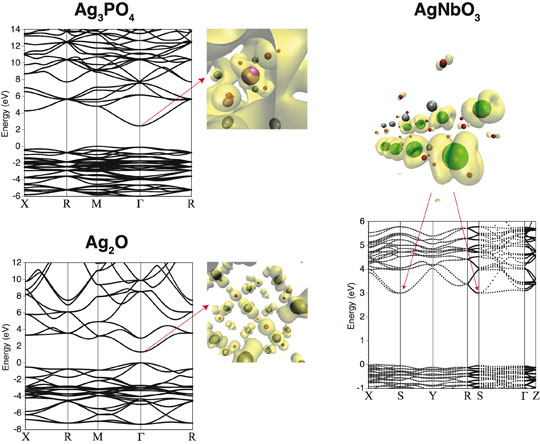
Fig. 4: Band structures for Ag3PO4, Ag2O, and AgNbO3 along with partial charge density at the bottom of the conduction band.
The origin of the high photo-oxidation activity of silver phosphate has been studied. We performed first-principles calculations for silver phosphate (Ag3PO4), silver oxide (Ag2O), and silver niobate (AgNbO3) and succeeded in determining the unique electronic structure of Ag3PO4 by the comparison of these silver compounds.
The band structures of these silver-based oxides are indicated in Figure 4. For Ag3PO4, it can be understood that the bottom of the conduction band is widely dispersed, because it is formed by an Ag s orbital, and thus is advantageous for electron transfer. In the figure, the constant-level contour surface of the wave function for Ag3PO4 suggests that this state is itinerant, indicating that electrons are readily transferred in any direction, and is considered to contribute to the high photocatalytic activity.
In Ag3PO4, P and O are strongly bound, suppressing the hybridization of Ag d and O p. Therefore, virtually no localized level originating in the Ag d orbital appears at the bottom of the conduction band. Instead, it was found that a band with a large dispersion is formed owing to the hybridization of Ag s – Ag s, and the effective mass of electrons is small and isotropic in comparison with that in other silver-based photocatalysts. In contrast, the bottom of the conduction band of Ag2O is localized because an Ag d - O p anti-bonding state orbital has formed (Fig. 4(b)). In the case of AgNbO3, it can be understood that the d orbitals of Nb are distributed anisotropically, limiting the directions in which the electrons can move (Fig. 4(c)).
From these results, we concluded that the isotropic, itinerant band structure at the bottom of the conduction band of Ag3PO4 contributes to the high photocatalytic activity. Although the hole carriers are mainly related to the oxidation reaction, the recombination with holes is suppressed by the rapid movement of photoexcited electrons formed in the bulk to the surface. Therefore, if the effective mass of electrons can be decreased, it is considered that this will in effect accelerate the oxidation reaction. Moreover, a comparison of Ag3PO4 and AgNbO3 revealed that the effective mass of holes is also smaller in Ag3PO4, which contributes to the high oxidation activity.
Publications
— N. Umezawa, O. Shuxin, and J. Ye,
"Theoretical study of high photocatalytic performance of Ag3PO4," Phys. Rev. B 83, 035202 (1-8), 2011.
— P. Reunchan and N. Umezawa,
“Native defects and hydrogen impurities in Ag3PO4,” Phys. Rev. B 87, 245205 (1-5), 2013.
Presentations
— Naoto Umezawa, Adisak Boonchun, Pakpoom Reunchan, Shuxin Ouyang, and Junhua Ye,
"Theoretical study of photocatalysis from defect, interface, and surface physics," International Union of Materials Research Societies, September 2013, Qingdao, China (invited)
— Naoto Umezawa, Ouyang Shuxin, and Jinhua Ye,
"Theoretical study of an excellent photocatalyst Ag3PO4," International Union of Materials Research Society, September 2010, Qingdao, China (invited)
4. Theoretical model for band-gap narrowing in assembled nanoparticles
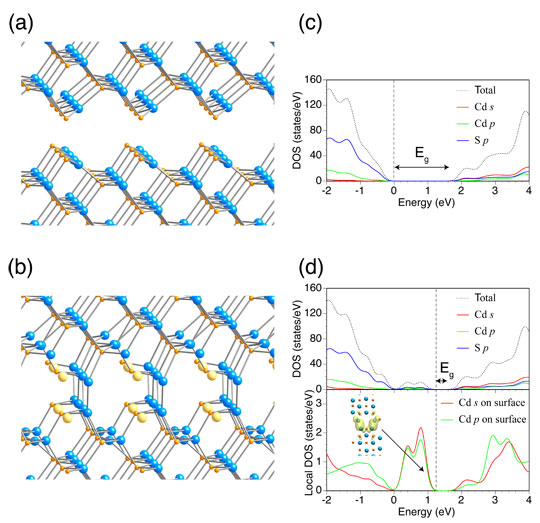
Fig. 5: Model of CdS surface contact with structural relaxation, showing (a) case where ideal surfaces of CdS (110) are arranged in mirror symmetry and (b) case where sulfur deficiency was introduced at the contact interface; (c) and (d) show the density of states (DOS) of the two cases, respectively. The figure shows the positions of atoms of Cd (blue) and S (orange) and sulfur deficiencies (yellow).
In order to form photocatalysts with high activity, it is necessary to amplify the absorption of visible light, which accounts for more than 40% of sunlight, by adjusting the band gap to an appropriate width. In this research, we attempted to develop a new type of visible-light-responsive photocatalyst by modifying the band structure by the aggregation of semiconductor nanoparticles.
The surface contact between nanoparticles was expressed by a simple model in which two surfaces were placed in confrontation, and the effect of aggregation was investigated theoretically. Figure 5(a) shows a model in which ideal the surfaces of CdS (110) are arranged with mirror symmetry, and Fig. 5(b) shows a model wherein sulfur deficiency is introduced at the contact interface in (a). According to density-functional theory calculations, structural relaxation results in the formation of a Cd-Cd bond at the contact interface when a sulfur deficiency is introduced (Fig. 5(b)).
The density of states (DOS) of the respective models is indicated in Fig. 5(c) and (d). In the case of the simple confrontation of the ideal surfaces, the same band gap as in the bulk was obtained (Fig. 5(c)). However, when the sulfur deficiency was introduced, a band associated with the interface was formed in the intrinsic band gap, effectively narrowing the band gap (Fig. 5(d)). From the density distribution of electrons occupying one of the gap states, it is understood that the newly formed band consists mainly of the Cd-Cd bonding states (Fig. 5(d)). The formation of the Cd-Cd bonds in the assembled nanoparticles was also supported by the calculations of the formation energy and experimental results, including XPS. The fact that visible light absorption is amplified by the aggregation of CdS nanoparticles, resulting in enhanced photocatalytic activity, was also confirmed experimentally, demonstrating the appropriateness of the model. A similar effect was observed with TiO2.
In the case of isolated nanoparticles, the band gap increases owing to the effect of quantum confinement (QC). However, in the case of aggregated nanoparticles, the band gap is determined by the competition between the QC effect and the bonding and anti-bonding states orbitals in the gap formed by surface contact between the particles. If the band gap of the aggregated nanoparticles can be controlled by the particle size of the constituent particles, this provides a simple technique for enhancing the visible light absorption efficiency. Therefore, we believe that this is an important finding that opens a new route in the band gap engineering of semiconductors.
Publications
— H. Tong, N. Umezawa, J. Ye, and T. Ohno,
"Electronic coupling assembly of semiconductor nanocrystals: self-narrowed band gap to promise solar energy utilization," Energy & Environmental Sci. 4, 1684-1689, 2011.
— H. Tong, N. Umezawa, and J. Ye,
"Visible light photoactivity from a bonding assembly of titanium oxide nanocrystals," Chem. Commun. 47, 4219-4221, 2011.
Presentations
— Naoto Umezawa and Jinhua Ye,
"Theoretical design of photocatalysts", International Union of Materials Research Society, September 2011, Taipei, Taiwan (invited).
5. Theory of photocatalytic oxidation reaction in N-doped TiO2
Nitrogen-doped TiO2 is a well-known visible-light sensitive photocatalyst wherein deep impurity states associated with substitutional nitrogen at oxygen sites (N0) are believed to be the source of the red shift in the photo-absorption edge. However, such a deep level should trap hole carriers, degrading the oxidation process. The contradiction between the deep N0 level and the rather high oxidation power of N-doped TiO2 remains an unsolved puzzle. Herein, we propose a convincing mechanism that successfully solves the riddle.
We performed a comprehensive theoretical analysis over the N-doped anatase TiO2, not only revealing the origin of the visible-light absorption but also providing a reasonable explanation of the photocatalytic oxidation reactions for N-doped TiO2. In this study, via density-functional theory plus an onsite Coulomb interaction (DFT+U), it was suggested that a substitutional nitrogen for oxygen (N0) prefers to bind with Ti atoms at interstitial sites (Tii), forming complex defects (No-Tii). This promotes the hybridization of N p with the Ti d states of Tii, giving rise to broader energy states at the valence band edge and eliminating the hole-trapping centers associated with the deep N0 states.
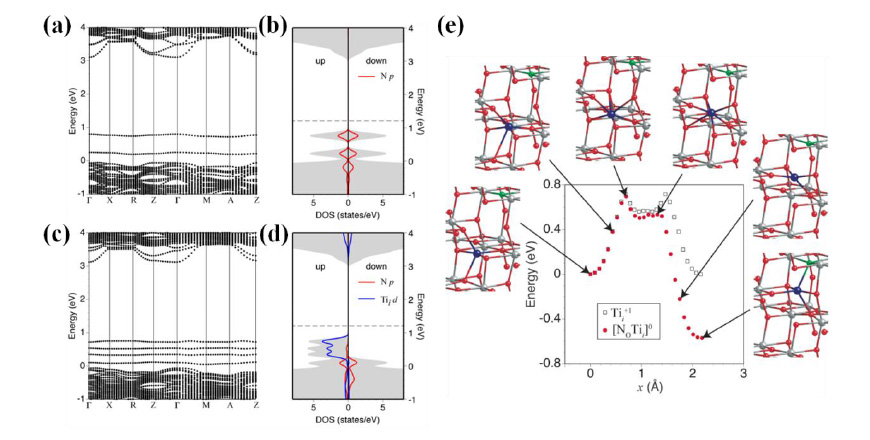
Fig. 6: Band structures of the up-spin state for (a) N0-1 and (b) [No-Tii]0 along highly symmetric points in the Brillouin zone associated with a 96-atom supercell. Total and local density of states (DOS) for the up- and down-spin states are also shown for (c) N0-1 and (d) [No-Tii]0. Here, the local DOS for Tii was integrated over the two nearest neighbor Ti atoms. The horizontal broken line indicates the highest occupied state. (e) Images of the intermediate configurations along the migration path of Tii+1 toward N0-1 and calculated total energies along the migration path compared with those without N0-1.
Figs. 6(a) and (c) indicate the band structures for the up-spin states of a negatively charged No-1 and a neutral [No-Tii]0, respectively. The deep impurity states associated with No-1 are replaced by multiple states above the valence band of the host TiO2 in the complex [No-Tii]0 (Fig. 6(c)) owing to the formation of bonding and anti-bonding states. The DOS in Fig. 6(d) indicates that these defect-impurity hybrid states in [No-Tii]0 are connected under an appropriate smearing parameter (0.1 eV) representing the thermal fluctuation. In contrast, the deep impurity state associated with No-1 is still disconnected from the valence band maximum of the host material (Fig. 6(b)), which is consistent with the previous report. The gap between the impurity state and the conduction band (2.4 eV) for [No-Tii]0 agrees well with the experimental data. This scenario is further supported by the migration property of Tii, as shown in Fig. 6(e). Notably, the second barrier in the migration path of Tii+1 disappears when it migrates toward N0-1, and the total energy monotonically decreases until it forms the complex [No-Tii]0. The estimated migration barrier is ~0.7 eV, which is readily overcome under a typical annealing temperature around 550 °C. This work offers a reasonable explanation for both the visible-light-driven and good photo-oxidation abilities of N-doped TiO2.
Publications
— N. Umezawa and J. Ye
"Role of complex defects in photocatalytic activities of nitrogen-doped anatase TiO2," Phys. Chem. Chem. Phys., 14, 5924-5934, 2012.
Presentations
— Naoto Umezawa, Adisak Boonchun, and Jinhua Ye,
"Theoretical Study of Native Defects and Doped Nitrogen in Anatase TiO2," 2013 Materials Research Society Spring Meeting, April 2013, San Francisco CA, USA.
— Naoto Umezawa and Jinhua Ye,
"Revisiting the mechanism of photocatalytic activities in N-doped TiO2," American Physical Society, March Meeting, February, 2012, Boston, USA.










