O2 Adsorption on a Platinum Surface Is Greatly Influenced by Its Molecular Alignment Relative to the Surface
— Clarification of a Key Factor Determining the Rate of Catalytic Oxidation Reactions —
National Institute for Materials Science (NIMS)
A NIMS research group has presented an alignment-controlled molecular oxygen adsorption experiment indicating that a catalytic oxidation process on Pt is strongly influenced by the geometry of an impinging O2 molecule relative to the surface.
("Dynamics of O
2 Chemisorption on a Flat Platinum Surface Probed by an Alignment-Controlled O
2 Beam", Hirokazu Ueta,
Mitsunori Kurahashi, Angew Chem Int Ed Engl. 2017 Apr 3;56(15):4174-4177., doi:
10.1002/anie.201612281)
Abstract
- A NIMS research group consisting of Mitsunori Kurahashi, a chief researcher at the RCAMC, and Ueta Hirokazu, ICYS researcher, has presented an alignment-controlled O2 adsorption experiment indicating that a catalytic oxidation process on Pt is strongly influenced by the geometry of an impinging O2 molecule relative to the surface. The present study has demonstrated the effectiveness of the O2 alignment-controlled experiment for understanding the mechanism of catalytic oxidation processes.
- O2 adsorption on Pt surfaces has attracted much attention since it is the initial step of catalytic oxidation reactions such as the car exhaust gas purification and the oxygen reduction reaction on a fuel cell electrode. The O2 sticking probability on flat Pt surfaces is however known to be much lower than that on surfaces of other precious metals such as palladium, rhodium and ruthenium. In addition, it shows almost energy-independent value of ~25% and does not increase even if the O2 translational energy is raised over 0.5 eV. These behaviors are considered to be closely associated with the low catalytic activity of flat Pt surfaces, but their origins remained unclear.
- The researchers in this group have clarified that O2 sticking probability on Pt(111) depends strongly on the geometry of an impinging O2 molecule relative to the surface, using the alignment-controlled O2 beam developed by them. They have demonstrated that only O2 molecules nearly parallel to the surface can adsorb at kinetic energy of ≤0.2 eV. This strong geometrical requirement for adsorption accounts for the low sticking probability of low energy O2 molecules. They have also clarified that the sticking probability for parallel O2 molecules decreases with energy at ≥0.5 eV while that for perpendicular molecules increases. The competing contributions of differently aligned molecules explain the almost energy-independent behavior of the sticking probability at high energy conditions.
- The present result means that the rate of catalytic oxidation reactions is strongly affected by the geometry of an impinging O2 molecule since the O2 sticking is one of the sequential steps involved in the whole catalytic process. The information obtained by the present analysis would help us to develop highly efficient catalysts and/or alternative materials containing a reduced amount of expensive precious metals.
- This study was conducted in conjunction with a project titled “Development of a high-energy-state-selected O2 beam and a highly sensitive measurement of alignment -dependent surface reactions,” funded by the JSPS Grant-in-Aid for Scientific Research (B). This study was also carried out in association with the NIMS 4th Mid-Term Program Project “Development of Advanced Characterization Technologies to Accelerate Materials Innovation”.
- This study was published in the online version of the German Chemical Society’s journal Angewandte Chemie International Edition, with recognition as a “very important paper,” on March 15, 2017, local time.
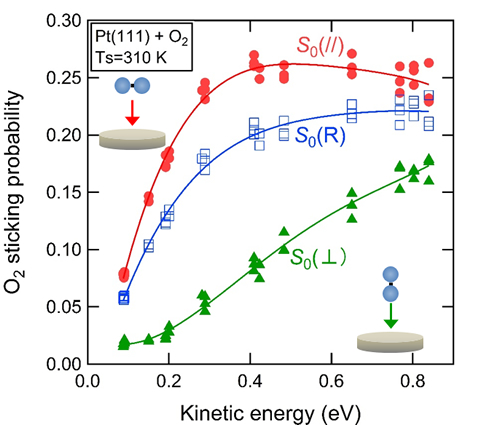
Figure 1 in the press release. The O2 sticking probability on a Pt(111) measured at different O2 alignment relative to the surface. (red: parallel, green: perpendicular, blue: random).