Discovery of Ferroelectric Structural Phase Transition in a Metal Substance That Was Predicted to Be Theoretically Possible About a Half Century Ago
2013.09.23
(2013.10.15 Update)
National Institute for Materials Science
Tohoku University
Dr. Kazunari Yamaura, Principal Researcher of the Strongly Correlated Materials Group in the Superconducting Properties Unit at NIMS, in a joint research with Dr. Andrew Boothroyd, Professor in the Physics Department of the University of Oxford, and Dr. Kenji Tsuda, Associate Professor at the Institute of Multidisciplinary Research for Advanced Materials of Tohoku University, succeeded in experimentally confirming structural phase transition that was predicted to be theoretically possible about a half century ago.
Abstract
- Dr. Kazunari Yamaura, Principal Researcher of the Strongly Correlated Materials Group in the Superconducting Properties Unit at the National Institute for Materials Science (NIMS; President: Sukekatsu Ushioda), in a joint research with Dr. Andrew Boothroyd, Professor in the Physics Department of the University of Oxford and Dr. Kenji Tsuda, Associate Professor at the Institute of Multidisciplinary Research for Advanced Materials of Tohoku University, succeeded in experimentally confirming structural phase transition that was predicted to be theoretically possible about a half century ago.
- Ferroelectricity is a property that microscopic electric dipoles (pairs of electric charges of equal magnitude but opposite sign, separated by a very small distance) in a crystal line up spontaneously, while involving a structural change, and the direction of the polarization can be reversed arbitrarily. Since the polarization can be reversed at normal temperature in some substances, ferroelectricity is useful for the development of ferroelectric memories and optical elements, and is applied widely in the device industry. In order to explore new uses and promote the development of next-generation devices, it is important to elucidate the mechanism of the spontaneous alignment and to develop a variety of ferroelectrics.
- Usually, when conduction electrons are present in a crystal, electric charges cannot stay on the crystal surface (dielectric polarization is not possible) since electric charges cannot become polarized within the crystal. Therefore, it was known that practical ferroelectrics are excellent electric insulators without exception. For achieving higher ferroelectricity, as few conduction electrons as possible should be present. However, a theoretical study in 1965 on structural phase transition by Nobel prize-winner Philip Anderson and co-worker Blount predicted that structural phase transition accompanying ferroelectricity can occur not only in electric insulators, but also in alloys and metallic compounds that have conduction electrons. This equivalent structural phase transition is referred to as “ferroelectric transition in a metal” for convenience. Following this theoretical study, continued attempts had been made to experimentally search for “ferroelectric transition in a metal,” but it remained unidentified for nearly 50 years.
- Dr. Yamaura and his collaborators focused on lithium niobate (LiNbO3) which was already used as a ferroelectric for extensive purposes, and searched for an unknown substance that had the same crystal structure and conduction electrons as lithium niobate. As a result, they succeeded in synthesizing lithium osmate (LiOsO3) under high temperature and pressure, and confirmed that lithium osmate had the same crystal structure and conduction electrons as lithium niobate, and that it undergoes structural phase transition equivalent to ferroelectric transition. Consequently, they succeeded in experimentally showing “ferroelectric transition in a metal,” which had been considered only theoretically possible, after a half century from the prediction in 1965.
- In-depth study of this equivalent structural phase transition will not only deepen universal understanding of the mechanism of spontaneous alignment of electric dipoles (i.e. ferroelectric structural phase transition), but also lead to new development in research. For instance, an unknown substance with a strong combination of structural transition and conduction electrons that derives ferroelectricity could be used as a high-temperature superconductor. The results of this research were achieved as part of the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program) ”Exploration of new superconductors and related functional materials and application of superconducting wires for industry” (core researcher: Hideo Hosono) of the Japan Society for the Promotion of Science. The results will be published in the online edition of the UK journal, Nature Materials, at 2:00 a.m., September 23, 2013 (JST).
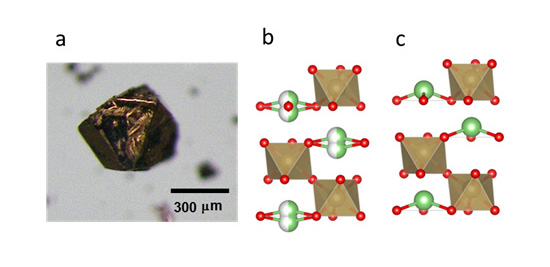
Figure: (a) Optical micrograph of a lithium osmate crystal, and (b) schematic diagram of its crystal structure (at normal temperature and (c) at an ultralow temperature of -130°C or less). The green/white balls represent the average positions of thermally disordered lithium ions (separated into two); the green balls represent lithium ions, red balls represent oxygen ions, and an osmium ion exists at the center of each octahedron.