Development of Nano-Captor for Removal of Iodine and Strontium in Polluted Water
Enables Selective Removal from Polluted Water Containing Chlorine and Minerals; High Expectations for Use in Nuclear Power Plant Treatment
2011.07.27
(2011.08.10 Update)
National Institute for Materials Science
Prof. Sherif A. El-Safty, a Senior Researcher of the Materials Circulation Design Group, Research Center for Strategic Materials, developed high-ordered mesoporous monolith (HOM) nano-captors which can selectively collect and remove trace amounts of iodine (I2) and strontium (Sr) in aqueous solutions.
Abstract
- Prof. Sherif A. El-Safty, a Senior Researcher of the Materials Circulation Design Group (Group Leader: Kohmei Halada) of the Research Center for Strategic Materials (Managing Director: Kaneaki Tsuzaki), National Institute for Materials Science (President: Sukekatsu Ushioda), developed high-ordered mesoporous (HOM) nano-captors that can selectively collect and remove trace amounts of iodine (I2) and strontium (Sr) in aqueous solutions.
- The newly-developed nano-captors are based on the utilization of highly ordered mesoporous silica monoliths (HOM) as carriers. These HOM carriers have extremely large number of unique nanopores on the surface and high surface area and pore volumes. In the development of nano-captors, a dense coating of adsorbent compounds was achieved inside the HOM nanopores. The selective capture of iodine or strontium was successfully achieved by mixing the prepared nano-captor in polluted water. Because, the adsorbent compounds covering the inner walls of the pores in the HOM nano-captor are arranged very densely, it is possible to capture even trace amounts of 0.001 ppm of iodine and 0.5 ppm of strontium.
- The ion-capture mechanism was prescribed the chemical interactions between the nano-captors and adsorbates (I2 & Sr). In principle, the radioactive isotopes such as I131 or Sr90, which are similar chemical substances to these adsorbates, can also be adsorbed and captured in the same manner by the nano-captors. Our findings show that it is possible to capture and remove 0.02 g of iodine per 1 g of nano-captor. Assuming all of this, the captured I131 can be equivalent to 90 terabecquerels (90x1012 becquerels). Likewise, 13 mg of strontium can be collected and removed with 1 g of the strontium captor, and this is equivalent to 65 gigabequerels (65x109 bequerels) of radioactive Sr90.
- An important feature of the HOM nano-captor is its high selectivity. Many conventional adsorbents also adsorb these target elements or substances in the same family with higher or lower periods of times. However, these conventional adsorbents can not distinguish between these target elements and other components, in which are commonly contained in large quantity in seawater, etc. For example, the disturbance components are like chlorine in the case of iodine removal, and magnesium (Mg) and calcium (Ca) in the case of strontium removal. The newly-developed HOM nano-captor can strictly identify the target element from other substances and selectively capture only the former. This means that iodine and strontium can be removed from aqueous solutions containing mixed seawater or mineral components.
- Furthermore, the iodine or strontium captured in the HOM nano-captor can be separated by the chemical treatment process, in which the stripping agent was used to release the elements or substances. This feature enables more effective management by concentrating the collected radioactive elements, thereby reducing the volume of material to be controlled, and the HOM nano-captor can be recycled repeatedly.
- As an additional feature, the iodine captor changes color when iodine is captured. Therefore, the fact is that capture process is being performed effectively and can be confirmed visually. Indeed, the HOM nano-captors can also be used in the detection of radioactive iodine.
- In the future, degradation tests, etc. will be carried out in radiation environments in collaboration with other organizations aiming at early supply to practical application.
- Part of the work on iodine in this research was carried out in the project “Development of technologies for removal of radioactive substances in soil in agricultural areas, etc. (development of recovery/removal technologies, etc. for radioactive substances in the environment using natural minerals and other inorganic materials)” under Ministry of Education, Culture, Sports, Science and Technology (MEXT) Aid for Science and Technology Strategy for FY2011 “Flexible response to important policy issues.”
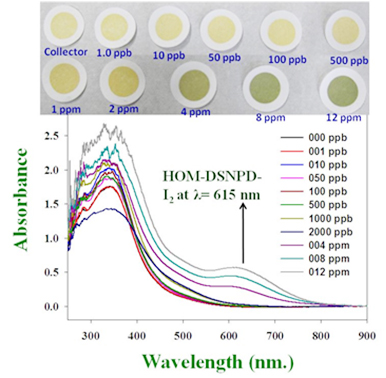
Figure: Change in HOM-DSNDP nano-captor with iodine concentration and the representative optical spectra and the difference in color map.